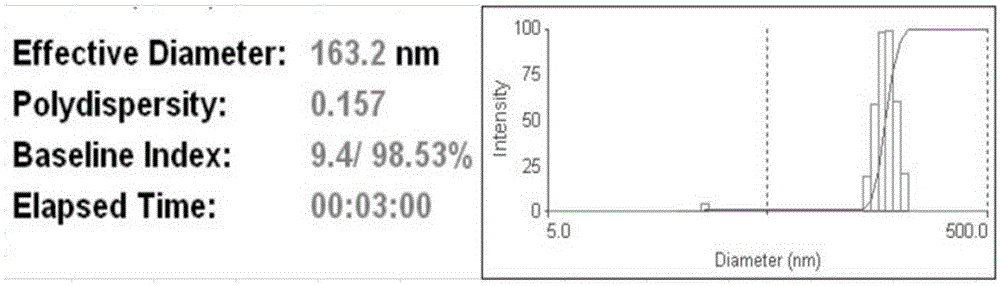
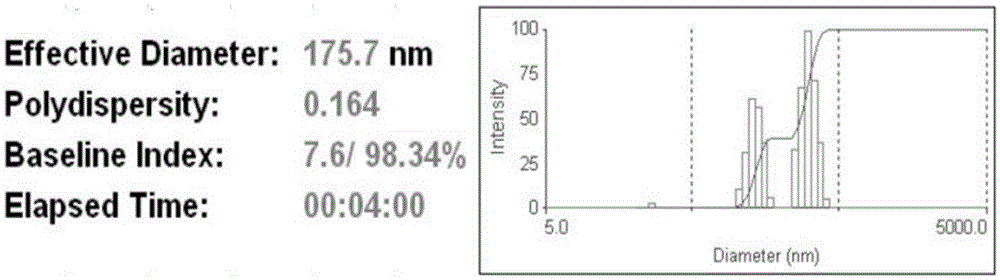
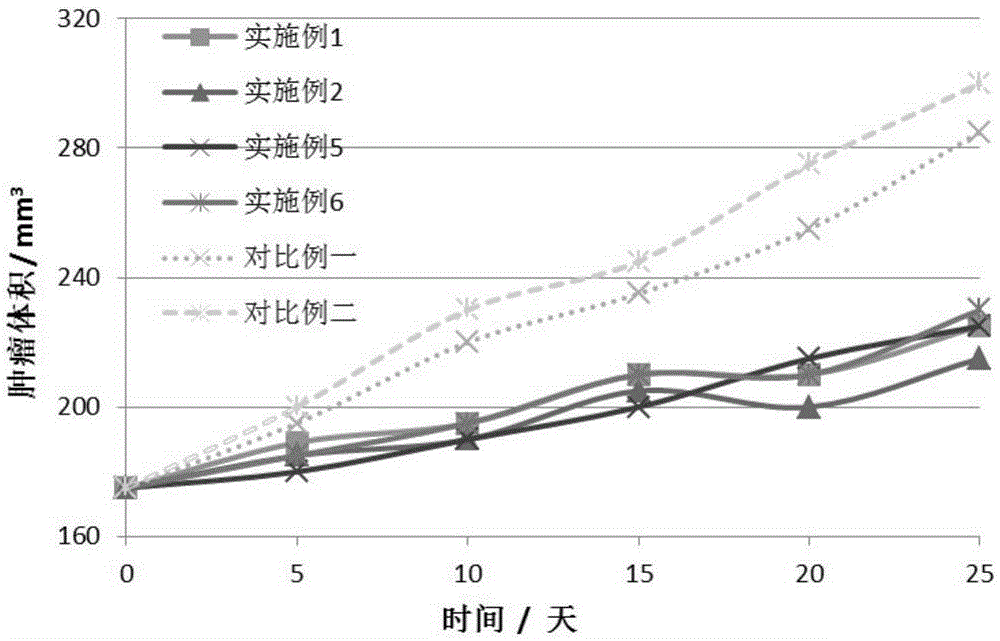
Targeted hydrophobic antitumor drug nano preparation and preparation method thereof
A technology of anti-tumor drugs and nano preparations, applied in the field of medicine, can solve the problems of reduced drug content in filtrate, reduced product yield, large particle size distribution coefficient, etc., to achieve enhanced inhibitory effect, ensure blood drug concentration, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of paclitaxel-targeted anti-tumor nano-preparation
[0047] (1) Preparation of hyaluronic acid-albumin conjugate:
[0048] Add 2.7042g of hyaluronic acid (molecular weight 2kDa) and 4.4958g of human serum albumin (the molar number is 1 / 20 times of hyaluronic acid) into 27.0mL of sterile water to dissolve, adjust the pH of the solution to 5.5, then mix 12.9600g of EDCI and 29. Add 3584g Sulfo-NHS to the above solution to react for 15min, adjust the pH of the solution to 7.4, continue to stir and react at room temperature for 5h, after the reaction is completed, dialyze to remove unbonded hyaluronic acid, unreacted EDCI, Sulfo-NHS and other By-products, hyaluronic acid-albumin conjugates were obtained after freeze-drying;
[0049] (2) Preparation of carrier solution:
[0050] Dissolve 0.9720g of human serum albumin and 0.1080g of hyaluronic acid-albumin conjugate in 216.0mL of sterile water to obtain a carrier solution;
[0051] (3) Preparation of...
Embodiment 2
[0055] Example 2 Preparation of paclitaxel-targeted anti-tumor nano-preparation
[0056] (1) Preparation of hyaluronic acid-albumin conjugate:
[0057] Add 2.4575g of hyaluronic acid (molecular weight 3kDa) and 8.1711g of human serum albumin (3 / 20 times the molarity of hyaluronic acid) into 27.0mL0.01MMES solution to dissolve, adjust the pH of the solution to 5.5, and then add 6.2813g of EDCI Add 14.2291g of Sulfo-NHS to the above solution to react for 15min, adjust the pH of the solution to 7.4, continue to stir and react at room temperature for 4h, after the reaction is completed, dialyze to remove unbonded hyaluronic acid, unreacted EDCI, Sulfo-NHS and Other by-products, hyaluronic acid-albumin conjugates are obtained after freeze-drying;
[0058] (2) Preparation of carrier solution:
[0059] Dissolve 1.2754g of human serum albumin and 0.3189g of hyaluronic acid-albumin conjugate in 213.0mL of sterile water to obtain a carrier solution;
[0060] (3) Preparation of drug s...
Embodiment 3
[0064] Example 3 Preparation of paclitaxel-targeted anti-tumor nano-preparation
[0065] (1) Preparation of hyaluronic acid-albumin conjugate:
[0066] Add 1.4319g of hyaluronic acid (molecular weight 5kDa) and 3.1741g of human serum albumin (1 / 6 times the molarity of hyaluronic acid) into 18.0mL0.01MPBS to dissolve, adjust the pH of the solution to 5.4, then mix 1.6470g of EDCI and 3. Add 3579g Sulfo-NHS to the above solution to react for 15min, adjust the pH of the solution to 7.5, continue to stir and react at room temperature for 4h, after the reaction is completed, dialyze to remove unbonded hyaluronic acid, unreacted EDCI, Sulfo-NHS and other By-products, hyaluronic acid-albumin conjugates were obtained after freeze-drying;
[0067] (2) Preparation of carrier solution:
[0068] Dissolve 1.0594g of human serum albumin and 0.4606g of hyaluronic acid-albumin conjugate in 150.0mL of sterile water to obtain a carrier solution;
[0069] (3) Preparation of drug solution:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com