Ferrocene bonding chromatographic stationary phase and preparing method thereof
A chromatographic stationary phase and ferrocene technology, applied in the field of liquid chromatography, can solve the problems of high cost and complicated process, and achieve the effects of simple operation, simple preparation process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
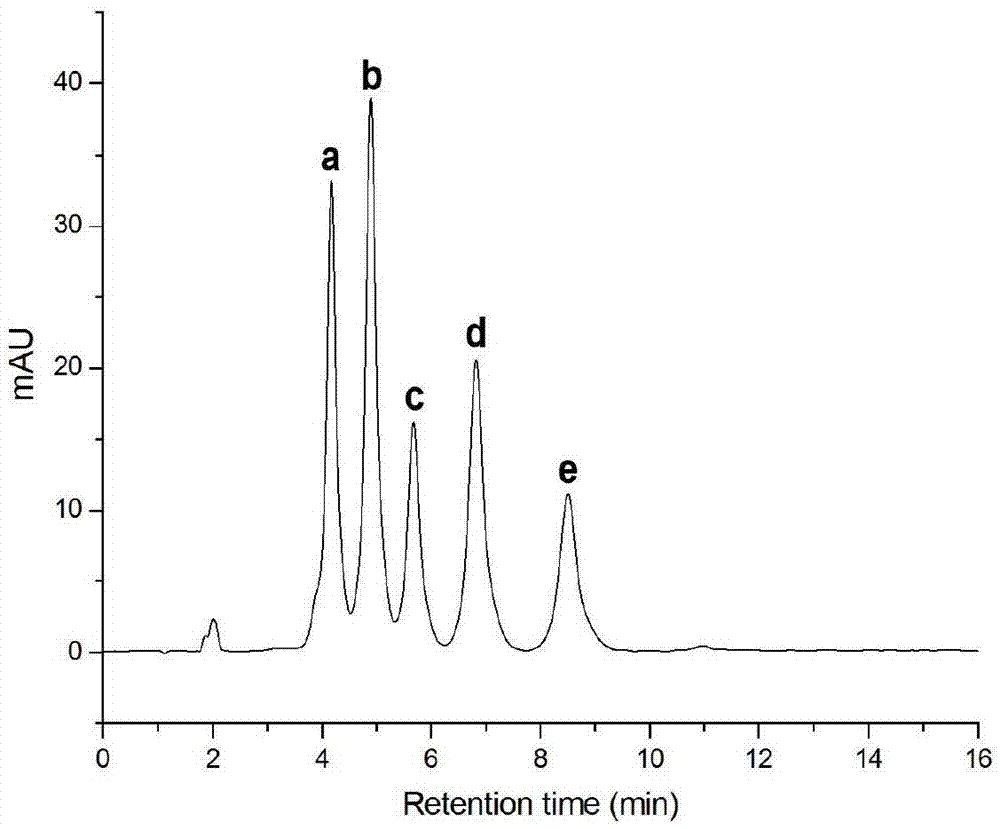
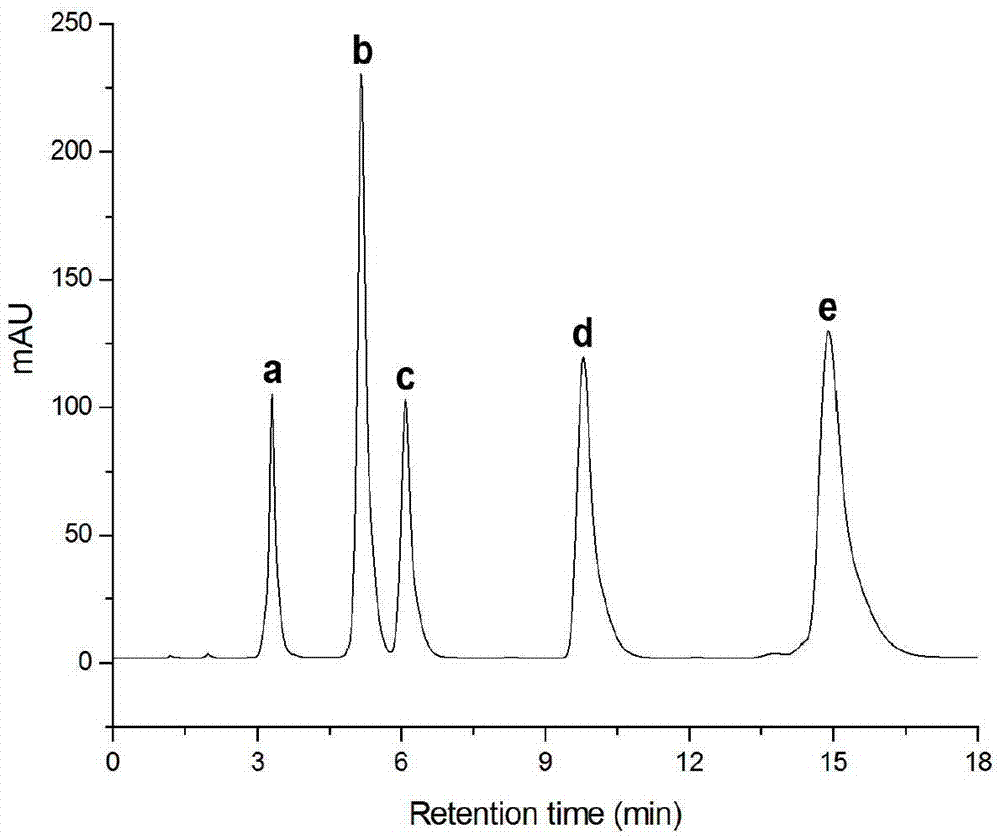
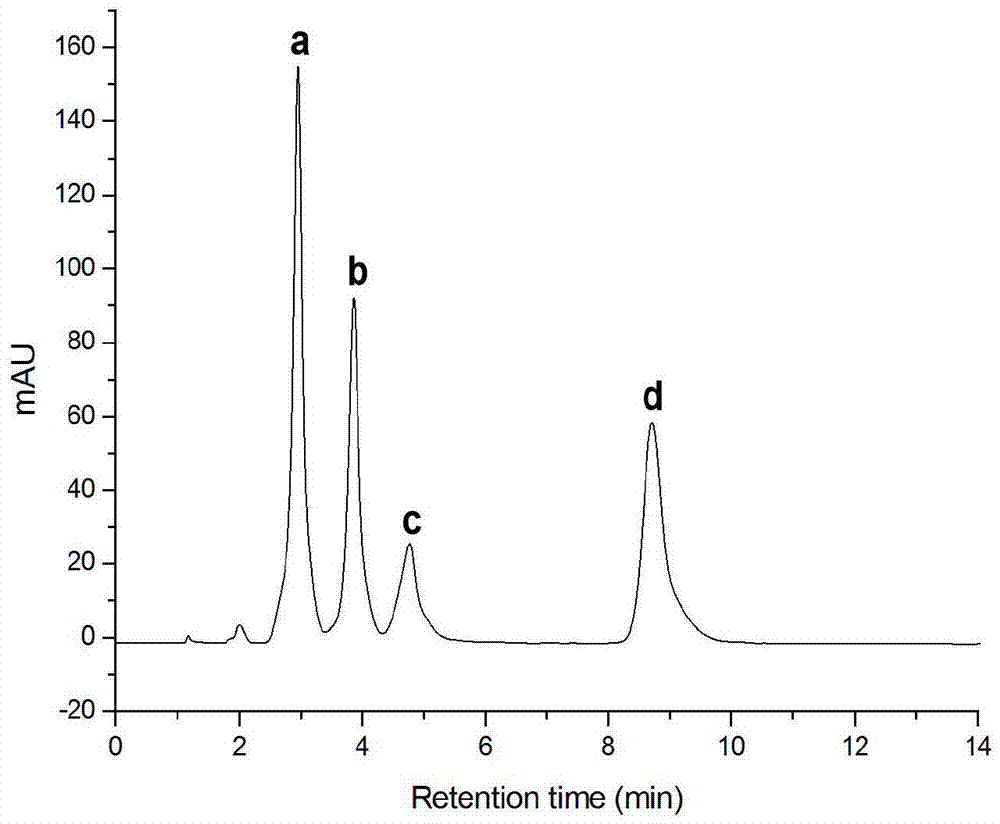
Image
Examples
Embodiment 1
[0025] Take 5.0g of activated silica gel (particle size 5um), add 150mL of freshly distilled anhydrous toluene to a 250mL round-bottomed three-neck flask, blow nitrogen for 30min, add 5mL of 3-mercaptopropyltrimethoxysilane under stirring, and add 3 drops of Triethylamine, under the protection of nitrogen, heat up to 110-120°C, stir and reflux for 24 hours, cool to room temperature, filter dry, wash with toluene, ether, acetone, methanol, acetone respectively for 2-3 times, and vacuum-dry at 60°C After 18 hours, 3-mercaptopropyl silica gel was obtained.
[0026] Take 4.5g of 3-mercaptopropylated silica gel and add it to 150mL of anhydrous toluene. Under stirring conditions, add 6.0g of vinyl ferrocene and 0.3g of AIBN respectively. Under the protection of nitrogen, heat the oil bath to 110°C, and reflux for 24h. The obtained product was washed successively with toluene, ethanol and acetone, and vacuum-dried at 80°C to obtain a ferrocene-bonded silica gel chromatography station...
Embodiment 2
[0029] According to the method for synthesizing 3-mercaptopropyl silica gel in Example 1, 5 g of activated titanium oxide microspheres (with a particle diameter of 7.5 μm) were used as a matrix filler to obtain 3-mercaptopropyl titanium oxide microspheres.
[0030] Take 4.5g of 3-mercaptopropylated titanium oxide microspheres and add them to 150mL of anhydrous toluene. Under stirring conditions, add 9.0g of vinylferrocene and 0.3g of AIBN respectively. Under the protection of nitrogen, heat the oil bath to 80°C and react After 36 hours, the obtained product was washed successively with toluene, ethanol and acetone, and dried under vacuum at 80°C to obtain a ferrocene-bonded titanium oxide chromatographic stationary phase. The stationary phase structure is:
[0031]
Embodiment 3
[0033] According to the method for synthesizing 3-mercaptopropyl silica gel in Example 1, take 5g of activated zirconia microspheres (particle size 10.0um) as matrix filler, and replace 3-mercaptopropyl trimethoxysilane with 3-mercaptopropyl triethyl Oxysilane can be used to prepare 3-mercaptopropyl zirconia microspheres.
[0034] Add 4.5g of 3-mercaptopropylated zirconia microspheres into 150mL of anhydrous toluene, add 13.5g of vinyl ferrocene and 0.3g of AIBN respectively under stirring conditions, heat the oil bath to 120°C under the protection of nitrogen, and reflux After 12 hours, the obtained product was washed successively with toluene, ethanol and acetone, and dried under vacuum at 80°C to obtain a ferrocene-bonded zirconia stationary phase for chromatography. The stationary phase structure is:
[0035]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com