Alkaline-lipase-producing Aspergillus niger mutant strain
A technology of mutant strain, Aspergillus niger, applied in the field of genetic engineering, can solve the problems of late alkaline lipase, lack of competitiveness and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The acquisition of embodiment 1 alkaline lipase gene
[0016] According to the gene sequence in the public gene database, the alkaline lipase gene LipN (sequence is SEQ ID NO: 2) was synthesized by Shanghai Jierui Company, and the amino acid sequence encoded by it is SEQ ID NO: 1.
[0017] PCR primers and reaction conditions are as follows:
[0018] Primer 1 (F): ATGCGGTCCTCCCTGGTGCTG
[0019] Primer 2 (R): TCACAGACAGGTGCCGATCAG
[0020] The reaction conditions were: denaturation at 94°C for 5 minutes; then denaturation at 94°C for 30 s, renaturation at 56°C for 30 s, extension at 72°C for 45 s, and after 30 cycles, incubation at 72°C for 10 min. The results of agarose electrophoresis showed that the size of the LipN gene was 876bp.
Embodiment 2
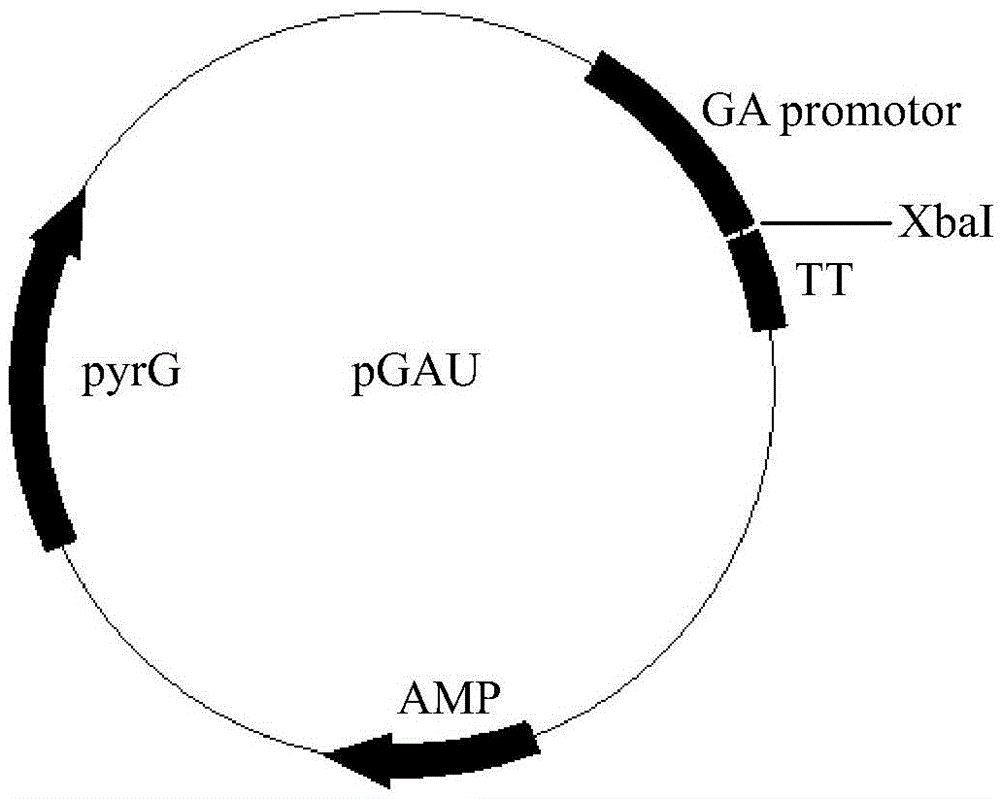
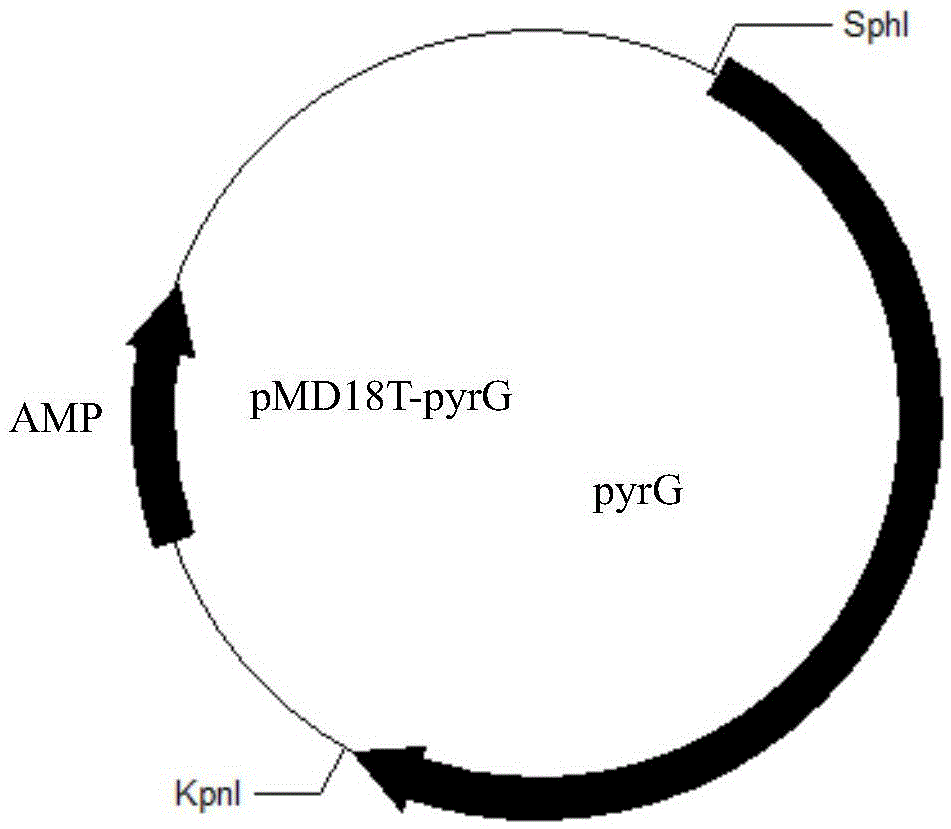
[0021] Embodiment 2 recombinant vector construction
[0022] The alkaline lipase gene was amplified by PCR, and XbaI sites were introduced at both ends of the primers. The primer sequences are as follows:
[0023] Primer 3 (F): GC TCTAGA ATGCGGTCCTCCCTGGTGCTG
[0024] Primer 4 (R): GC TCTAGA TCACAGACAGGTGCCGATCAG
[0025] The PCR reaction conditions were: denaturation at 94°C for 5 minutes; then denaturation at 94°C for 30 seconds, renaturation at 56°C for 30 seconds, extension at 72°C for 45 seconds, and after 30 cycles, incubation at 72°C for 10 minutes. The results of agarose gel electrophoresis showed that the LipN gene was a fragment with a size of 876bp.
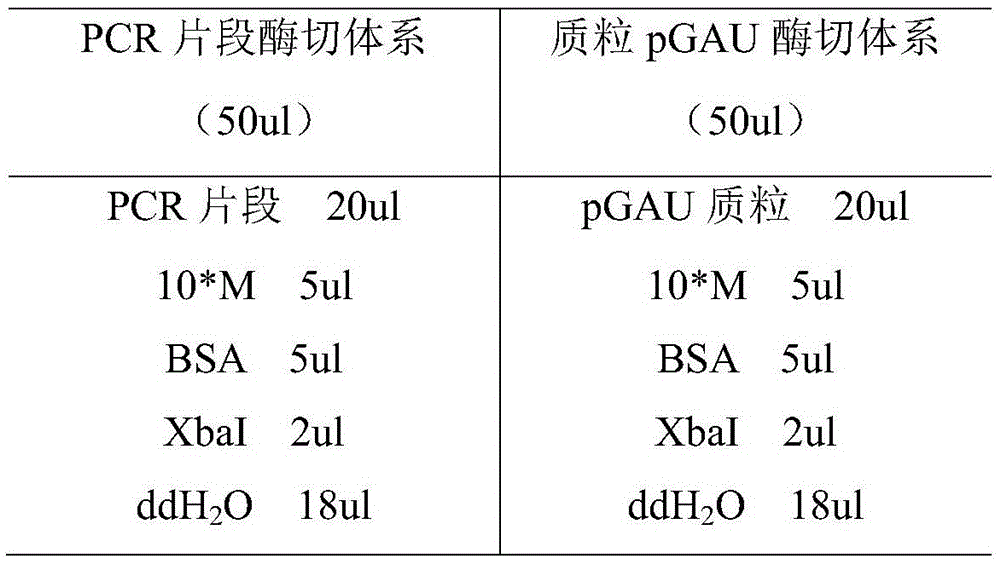
[0026] The alkaline lipase LipN fragment obtained above and the expression vector pGAU were subjected to single digestion with restriction endonuclease XbaI respectively, and the digestion conditions were as follows:
[0027]
[0028] Enzyme digestion treatment in water bath at 37°C for 2 hours, after electr...
Embodiment 3
[0031] The recombinant expression of embodiment 3 alkaline lipase LipN
[0032] Protoplast preparation: inoculate Aspergillus niger host Su2-1 on PDA+U plates, and culture at 30°C for 5-7d. Bacterial blocks with a size of 2cm×2cm were cut out, inoculated into 100ml liquid PDA+U medium, and cultured at 30°C for 24h to grow mycelia for transformation. After filtering the grown mycelia, resuspend with 20ml of 1.2M magnesium sulfate solution, and add 0.2g of lysozyme. Cultivate at 30°C and 100rpm for 2-3h. The lysed hyphae were filtered with two layers of lens paper, and centrifuged at 3000rpm for 10min to obtain protoplasts.
[0033] Transformation: the protoplasts were washed twice with 1.2M sorbitol solution, and then resuspended with an appropriate amount of sorbitol solution to make the protoplast concentration reach 10 8 . Add 10ul prepared plasmid to 200ul protoplasts, add 50ul 25% PEG6000, ice-bath for 20min, then add 2ml25% PEG6000 and place at room temperature for 5m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com