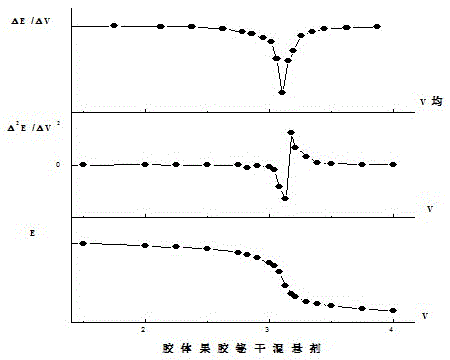
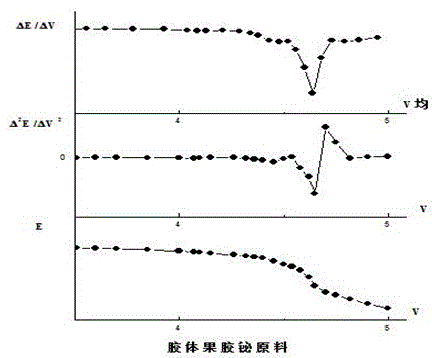
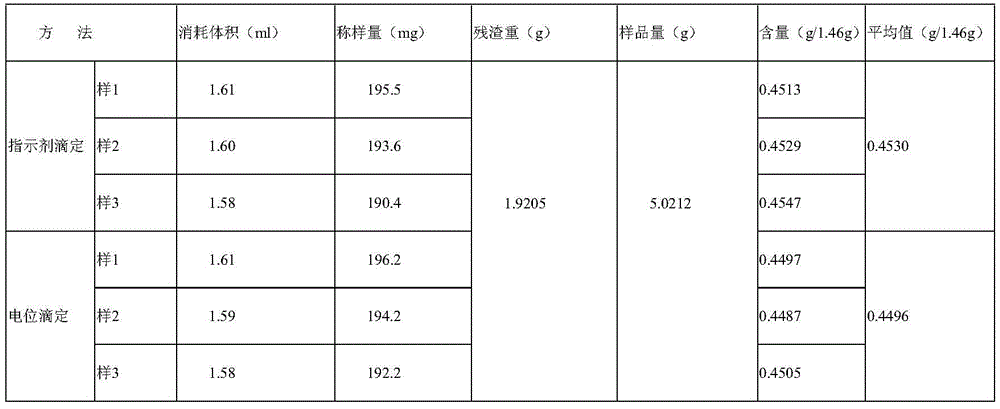
Method for controlling quality of colloidal bismuth pectin medicine composition and measuring galacturonic acid content of colloidal bismuth pectin medicine composition
A quality control method and technology of colloidal bismuth pectin, which are applied in the field of determination of galacturonic acid content, can solve the problems such as the inability to further improve the curative effect of colloidal bismuth pectin, the quality control is not strict enough, the product controllability is poor, etc. The standard is scientific, reasonable and controllable, the effect of enhancing the dehydration effect and reducing the drying time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0036] 1. Preparation of colloidal bismuth pectin:
[0037] (1) Add 166.67g of purified water into the reaction flask, add 29.22g of bismuth nitrate; add about 23.78g of 40% potassium hydroxide solution, adjust the pH to 6-8, and make it hydrolyze completely (that is, retest the pH to be constant) Finally, filter to get the filter cake bismuth hydroxide; then put 40.56g of purified water and 17.89g of sorbitol into the beaker, and then add the filter cake bismuth hydroxide after the sorbitol dissolves, and add 40% potassium hydroxide after stirring About 50g of the solution, stir to make it fully dissolve, and obtain the bismuth salt solution for later use;
[0038] In (2), add the bismuth salt solution prepared in step (1) into the reaction bottle, stir, add 49ml of purified water, add pectin soft material at room temperature, after the addition is completed, heat up to about 40°C and stir, and keep the temperature for 0.5 hours. Then add 105g of purified water, control the ...
Embodiment 2
[0040] Embodiment 2, the preparation of colloidal bismuth pectin dry suspension
[0041] Prescription: colloidal bismuth pectin 1000 (based on 150g bismuth)
[0042] Mannitol 455g
[0043] Disodium hydrogen phosphate 15g
[0044] Specification: 150mg (calculated as bismuth)
[0045]Production method: 1) Weigh the raw and auxiliary materials in the prescription on an electronic scale according to the ratio in the prescription; 2) Put the weighed colloidal bismuth pectin, disodium hydrogen phosphate and mannitol into the mixing machine in sequence Mixing, the mixing time is 30 minutes, and the speed of the total mixer is 50Hz; 3) After mixing, the product is sampled (about 30g) for detection, and after passing the test, it is divided into 1000 bags, vacuum-sealed, labeled, stored, wrap up and store.
Embodiment 3
[0046] The preparation of embodiment 3 colloidal bismuth pectin dry suspension
[0047] Prescription: colloidal bismuth pectin 900kg
[0048] Mannitol 409.5kg
[0049] Disodium hydrogen phosphate 13.5kg
[0050] Specification: 150mg (calculated as bismuth)
[0051] Production method: 1) Weigh the raw and auxiliary materials in the prescription on an electronic scale according to the ratio in the prescription; 2) Put the weighed colloidal bismuth pectin, disodium hydrogen phosphate and mannitol into the mixing machine in sequence Mixing, the mixing time is 30 minutes, and the speed of the total mixer is 50Hz; 3) After mixing, the product is sampled (about 30g) for detection, and after passing the test, it is divided into 900,000 bags, vacuum-sealed, labeled, and stored ,wrap up and store.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com