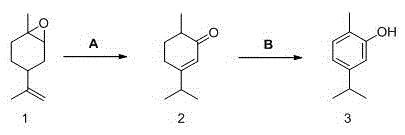
Method for synthesizing carvacrol by limo nene epoxides
A technology for epoxy limonene and carvacrol is applied in the chemical field, can solve problems such as limited application, environmental pollution, aluminum salt and iron salt solid waste generation, etc., and achieves the effects of convenient source, green synthesis process and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Step 1: Add 300g (2mol) of epoxy limonene and 5g (0.03mol) of cuprous bromide into a 500mL three-necked flask, and then react at 160°C for 6-8 hours. After the GC shows that the reaction is over, add 30g of white oil to the reaction. After vacuum distillation, 250 g of isodihydrocarvone with a content greater than 90% was obtained, with a yield of 83%. In addition, the rectification still liquid containing the catalyst can catalyze the reaction to proceed normally again.
[0022] Step 2 Put 250g (1.67mol) of isodihydrocarvone obtained above and 20g of xylene into a 500mL three-necked flask, then add 100g (0.05mol) of catalyst B, and react under reflux at 190-220°C. During the reaction, Constantly use the pump to blow air under the surface of the reaction liquid to continuously distill the water produced by dehydrogenation. After about 4 to 5 hours, GC shows that 39% of isodihydrocarvone remains, stop the reaction, filter to remove the catalyst, and decompress the filtra...
Embodiment 2
[0025] Step 1: Add 300 g (2 mol) of epoxylimonene and 3 g (0.02 mol) of cuprous bromide into a 500 mL three-necked flask, and then react at 170 ° C for about 4 to 5 hours. After the GC shows that the reaction is over, rectify under reduced pressure to obtain a content greater than 90% isodihydrocarvone 255g, productive rate 85%.
[0026] Step 2 Put 255g (1.7mol) of isodihydrocarvone obtained above and 20g of xylene into a 500mL three-necked flask, then add 50g (0.023mol) of catalyst B, and react under reflux at 190-220°C. During the reaction, Constantly use the pump to blow air under the reaction liquid surface, about 6 to 8 hours, GC shows that 40% of isodihydrocarvone remains, stop the reaction, filter to remove the catalyst, carry out vacuum distillation on the filtrate, recover 16g of solvent and unreacted 97g of raw materials were further rectified to obtain 118g of carvacrol finished product with a content greater than 98%, and the effective yield was 75%.
Embodiment 3
[0028] Step 1: Add 300g (2mol) of epoxy limonene and 3.5g (0.01mol) of nickel trifluoromethanesulfonate into a 500mL three-necked flask, and then react at 160°C for about 2 to 3 hours. After the reaction is shown by GC, rectify under reduced pressure 240 g of isodihydrocarvone with a content greater than 90% was obtained, and the yield was 80%.
[0029] Step 2 Put 240g (1.6mol) of isodihydrocarvone obtained above and 20g xylene into a 500mL three-necked flask, then add 80g (0.038mol) of catalyst B, and react under reflux at 190-220°C. During the reaction, Continuously use the pump to blow air under the reaction liquid surface, about 6 to 7 hours, GC shows that 32% of isodihydrocarvone remains, stop the reaction, filter to remove the catalyst, and carry out vacuum distillation on the filtrate to recover 16g of solvent and unreacted 76g of raw material was further rectified to obtain 118g of carvacrol finished product with a content greater than 98%, and the effective yield was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com