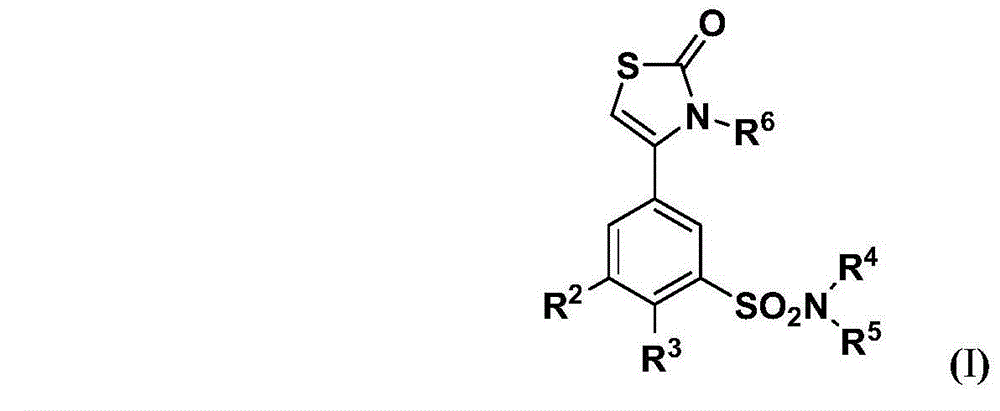
Dihydrothiazolone compounds containing sulfamide and pharmaceutical compositions and use thereof
A dihydrothiazolone and sulfonamide-containing technology, which can be used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
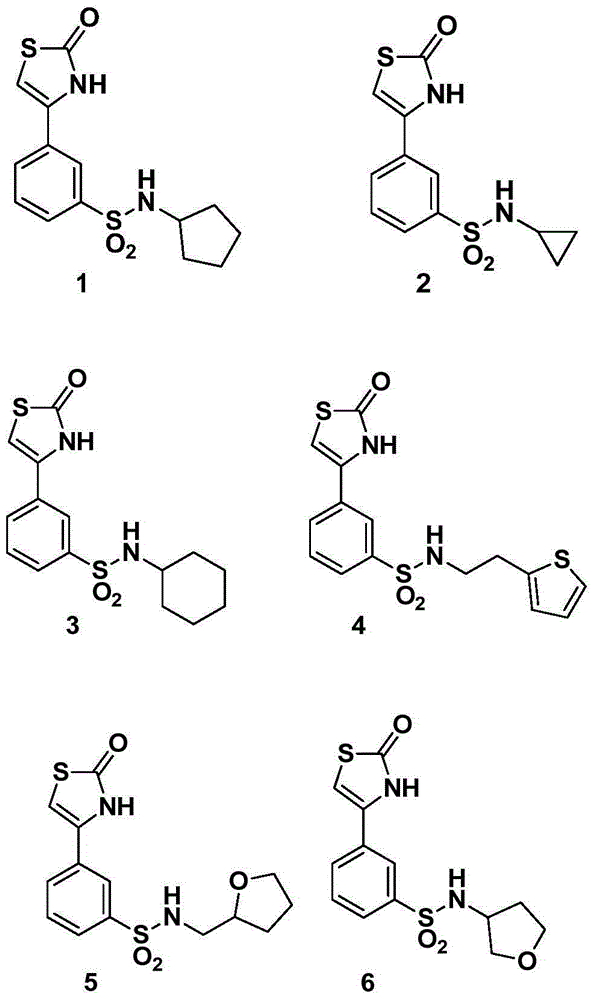
Image
Examples
Embodiment 1
[0116]
[0117] The synthetic route is:
[0118]
[0119] Reagents and conditions: a) sodium nitrite, cuprous chloride, concentrated hydrochloric acid, glacial acetic acid, water, saturated sulfur dioxide solution of glacial acetic acid, -15°C to room temperature; b) cyclopentylamine, pyridine, dichloromethane, room temperature; c) copper bromide, ethyl acetate, 80°C; d) potassium thiocyanate, acetone, room temperature; e) 50% sulfuric acid, glacial acetic acid, 100°C
[0120] a) 3-Aminoacetophenone (1g, 7.4mmol) was dissolved in 5mL of glacial acetic acid and 5mL of concentrated hydrochloric acid, sodium nitrite (0.613g, 8.88mmol) was dissolved in 2mL of water, and slowly added to the reaction solution at -15°C, Control the temperature not to be higher than -5°C, stir for 30 minutes; dissolve cuprous chloride (0.22g, 2.22mmol) in 10mL of saturated sulfur dioxide solution of glacial acetic acid, stir at 0°C for 30 minutes, the solution changes from dark green to blue-gre...
Embodiment 15
[0130]
[0131] The synthetic route is:
[0132]
[0133] Reagents and conditions: a) reduced iron powder, ammonium chloride, ethanol, water, 80°C; b) sodium nitrite, cuprous chloride, concentrated hydrochloric acid, glacial acetic acid, water, saturated sulfur dioxide solution of glacial acetic acid, -15°C to room temperature; c) cyclopentylamine, pyridine, dichloromethane, room temperature; d) copper bromide, ethyl acetate, 80 ° C; e) potassium thiocyanate, acetone, room temperature; f) 50% sulfuric acid, glacial acetic acid, 100°C
[0134] a) 3-nitro-4-methylacetophenone (3g, 16.74mmol) was dissolved in 20mL ethanol, ammonium chloride (3.58g, 67mmol) was dissolved in 5mL water and added to the reaction solution, and then reduced iron powder ( 3.74g, 67mmol), the reaction solution was heated to 80°C and stirred for 30 minutes, the reaction solution was diluted with ethyl acetate, filtered through diatomaceous earth, the filtrate was concentrated under reduced pressure...
Embodiment 43
[0139]
[0140] The synthetic route is:
[0141]
[0142] Reagents and conditions: a) reduced iron powder, ammonium chloride, ethanol, water, 80°C; b) sodium nitrite, cuprous chloride, concentrated hydrochloric acid, glacial acetic acid, water, saturated sulfur dioxide solution of glacial acetic acid, -15°C to room temperature; c) cyclopentylamine, pyridine, dichloromethane, room temperature; d) copper bromide, ethyl acetate, 80 ° C; e) potassium thiocyanate, acetone, room temperature; f) 50% sulfuric acid, glacial acetic acid, 100°C; g) ethyl 2-bromoacetate, potassium carbonate, potassium iodide, N,N-dimethylformamide, 100°C
[0143] Steps a, b, c, d, e, f were carried out in a similar manner to Preparation Example 15 to obtain Intermediate G. MS(ES):m / z339.10[M+H] + ; 1 HNMR(400MHz,DMSO)δ11.97(s,1H),8.09(s,1H),7.79–7.70(m,2H),7.45(d,J=7.8Hz,1H),6.86(s,1H), 3.44(dd,J=14.0,6.8Hz,1H),2.58(s,3H),1.53(m,4H),1.39–1.22(m,4H).
[0144] g) Intermediate G (150mg, 0.443mmol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com