Method for improving electrochemical performance of nickel-cobalt lithium manganate by chemically reducing oxidized graphene/magnesium
A technology of nickel-cobalt lithium manganese oxide and graphene, which is applied in the field of chemically reducing graphene oxide/magnesium to improve the electrochemical performance of nickel-cobalt lithium manganese oxide, can solve the problems of complex steps, many raw materials, long time, etc., and achieve low production cost, Improved performance and reduced processing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment includes the following steps:
[0034] Step 1. Prepare 300 mL of a mixed aqueous solution containing 0.01 g of graphene oxide and 0.1 g of magnesium chloride.
[0035] Step 2. Add 2 g of commercially available nickel-cobalt-lithium manganese oxide powder into the above mixed aqueous solution, and stir for 8 min at a stirring speed of 500 rpm.
[0036] Step 3. Wash the nickel-cobalt lithium manganate powder after the above reaction, filter it with water, and vacuum-dry it in a vacuum drying oven with a pressure less than -0.08Mpa to obtain nickel-cobalt with magnesium ions and a chemically reduced graphene oxide layer. Lithium manganate powder.
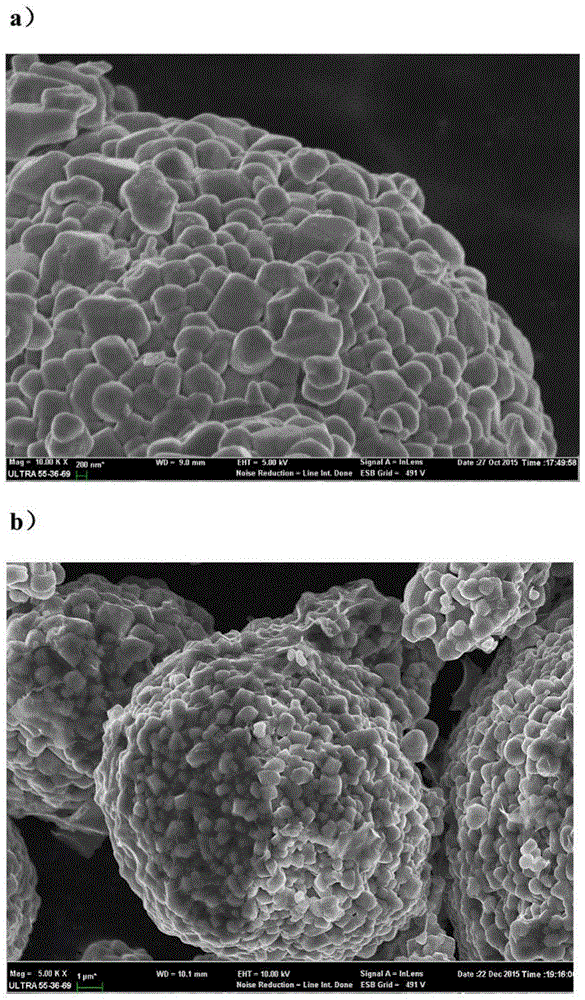
[0037] Such as figure 1 As shown, compared with the untreated nickel-cobalt-lithium-manganese-oxide powder (a), the surface of the treated nickel-cobalt-lithium-manganese-oxide powder has an obvious chemically reduced graphene oxide layer.
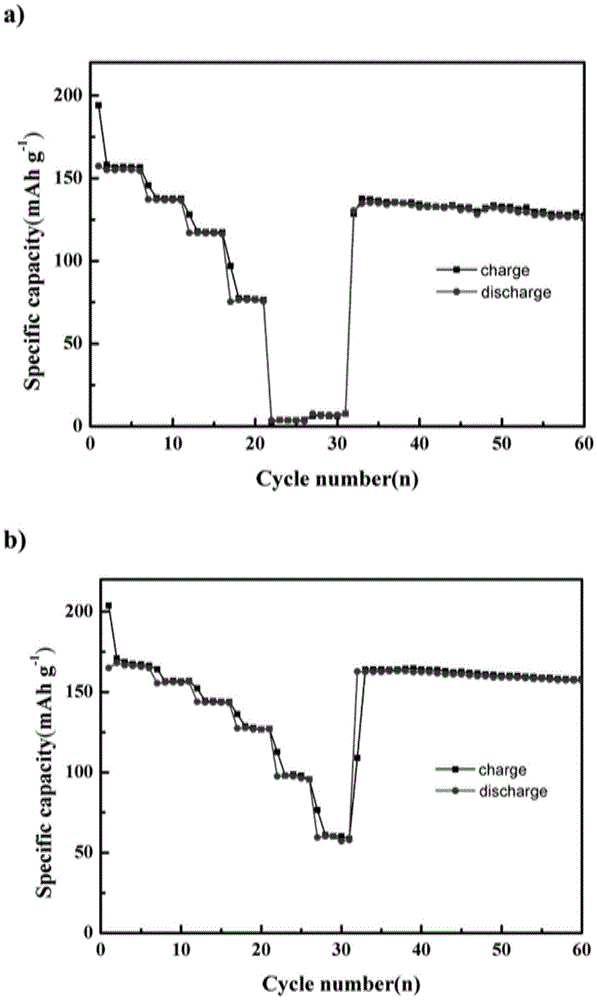
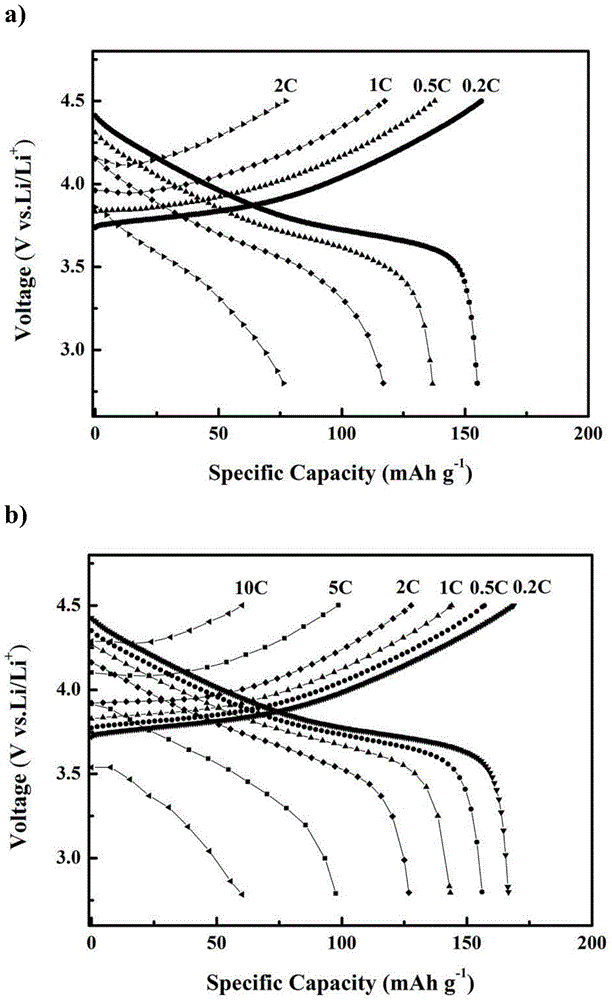
[0038] Such as figure 2 with image 3 As shown, the present embodi...
Embodiment 2
[0044] This embodiment includes the following steps:
[0045] Step 1. Prepare 200 mL of a mixed aqueous solution containing 0.01 g of graphene oxide and 0.15 g of magnesium chloride.
[0046] Step 2. Add 5 g of commercially available nickel-cobalt-lithium manganese oxide powder into the above mixed aqueous solution, and stir for 15 min at a stirring speed of 450 rpm.
[0047] Step 3. Wash the nickel-cobalt lithium manganate powder after the above reaction, filter it with water, and vacuum-dry it in a vacuum drying oven with a pressure less than -0.08Mpa to obtain nickel-cobalt with magnesium ions and a chemically reduced graphene oxide layer. Lithium manganate powder.
[0048] The nickel cobalt lithium manganese oxide powder before and after treatment was made into a lithium ion battery in the same manner as in Example 1, and then the charge and discharge performance test was carried out. The test results of charge and discharge performance show that: after the nickel cobalt l...
Embodiment 3
[0050] This embodiment includes the following steps:
[0051] Step 1. Prepare 300 mL of a mixed aqueous solution containing 0.02 g of graphene oxide and 0.08 g of magnesium sulfate.
[0052] Step 2. Add 1 g of commercially available nickel-cobalt-lithium manganese oxide powder into the above mixed aqueous solution, and stir for 8 minutes at a stirring speed of 500 rpm.
[0053] Step 3. Wash the nickel-cobalt lithium manganate powder after the above reaction, filter it with water, and vacuum-dry it in a vacuum drying oven with a pressure less than -0.08Mpa to obtain nickel-cobalt with magnesium ions and a chemically reduced graphene oxide layer. Lithium manganate powder.
[0054] The nickel cobalt lithium manganese oxide powder before and after treatment was made into a lithium ion battery in the same manner as in Example 1, and then the charge and discharge performance test was carried out. The test results of charge and discharge performance show that after the nickel cobal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com