Difluprednate ophthalmic emulsion and preparation method thereof
A technology for difluprednate and ophthalmic use, which is applied in the field of difluprednate-containing ophthalmic emulsion and its preparation, and can solve the problems of epithelial cell shedding and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
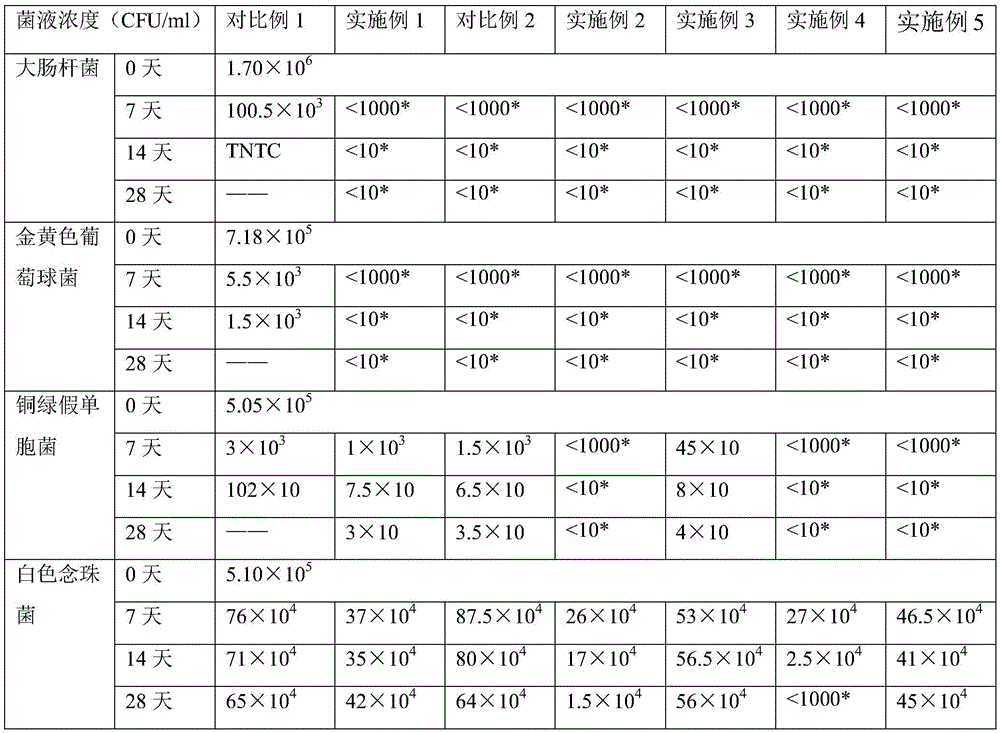
Examples
Embodiment 1
[0035] prescription:
[0036] Raw materials
Dosage
%w / v
difluprednate
0.5g
0.05
50g
5
Tween 80
40g
4
Glycerin
22g
2.2
Sodium acetate
0.5g
0.05
1g
0.1
0.01g
0.001
Appropriate amount
water
Add water to 1000ml
[0037] crafting process:
[0038] (1) Oil phase preparation: heat castor oil to about 70°C, add difluprednate, stir to dissolve, and use it as the oil phase.
[0039] (2) Water phase preparation: heat water to about 70° C., add Tween-80, glycerin, sodium acetate, boric acid, polyquaternium-1, stir to dissolve, and use as the water phase.
[0040] (3) Preparation of colostrum: add the oil phase obtained in the step (1) into the water phase obtained in the step (2), disperse at high speed for 10 minutes, and the shear rate is 10000 rpm to form colostrum a...
Embodiment 2
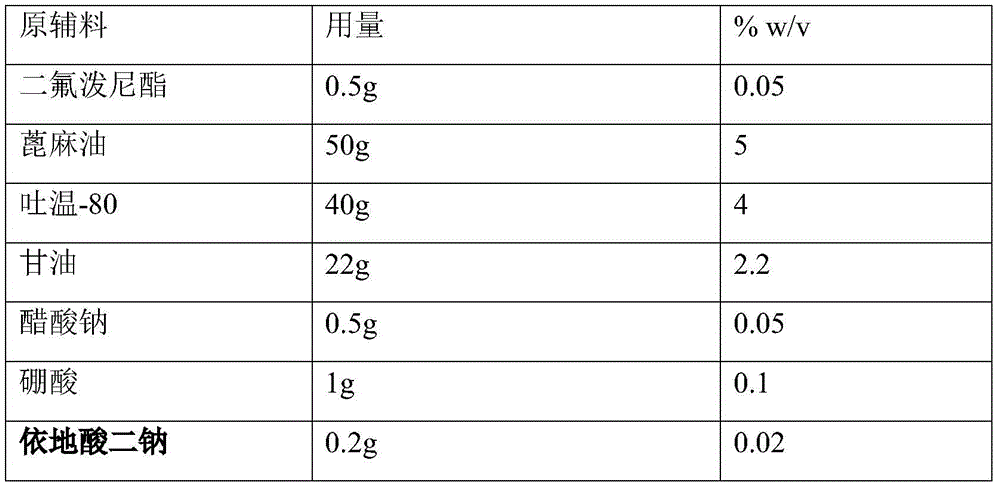
[0052] prescription:
[0053] Raw materials
Dosage
%w / v
difluprednate
0.5g
0.05
50g
5
Tween 80
40g
4
Glycerin
22g
2.2
Sodium acetate
0.5g
0.05
1g
0.1
0.2g
0.02
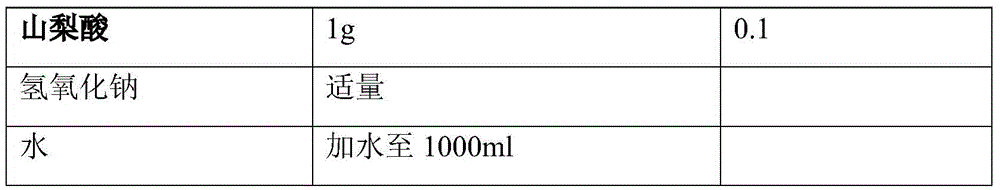
0.01g
0.001
Appropriate amount
4 -->
water
Add water to 1000ml
[0054] crafting process:
[0055] (5) Oil phase preparation: heat castor oil to about 70° C., add difluprednate, stir to dissolve, and use it as the oil phase.
[0056] (6) Preparation of water phase: Heat water to about 70°C, add Tween-80, glycerin, sodium acetate, boric acid, disodium edetate, polyquaternium-1, stir to dissolve, and use as water phase.
[0057] (7) Preparation of colostrum: add the oil phase obtained in the step (1) into the water phase obtained in the step (2), disperse at high speed for 1...
Embodiment 3
[0060] prescription:
[0061] Raw materials
Dosage
%w / v
difluprednate
0.5g
0.05
castor oil
50g
5
Tween 80
40g
4
Glycerin
22g
2.2
Sodium acetate
0.5g
0.05
boric acid
1g
0.1
0.2g
0.01
0.01g
0.0005
Appropriate amount
water
Add water to 1000ml
[0062] crafting process:
[0063] (1) Oil phase preparation: heat castor oil to about 70°C, add difluprednate, stir to dissolve, and use it as the oil phase.
[0064] (2) Preparation of water phase: Heat water to about 70°C, add Tween-80, glycerin, sodium acetate, boric acid, disodium edetate, polyquaternium-1, stir to dissolve, and use as water phase.
[0065] (3) Preparation of colostrum: add the oil phase obtained in the step (1) into the water phase obtained in the step (2), disperse at high speed for 10 mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com