Sacubitril dicyclohexylamine salt, crystal form and preparation method of crystal form
A technology of dicyclohexylamine salt and sacubitril, applied in the field of drug synthesis, can solve the problems of low production efficiency, unsatisfactory crystallization effect, unfavorable industrial purification and the like, and achieves reduction of production cost, easy production amplification, storage and circulation, Impurity control ideal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of the crude product of embodiment 1 sand library than tricyclohexylamine salt
[0040] At room temperature (10-30°C), add 4.5g of Sacubitril to 25mL of ethyl acetate, stir to dissolve, then slowly drop in 1.7g of dicyclohexylamine, continue to stir to form solids, and then cool down naturally to After 2 hours of heat preservation at 3°C, filter, wash the filter cake with ethyl acetate, and dry in vacuo to obtain 6.0 g of white powder, which is the crude product of sacubitril dicyclohexylamine salt.
Embodiment 2
[0041] The preparation of the crystalline form of embodiment 2 Sacubitril dicyclohexylamine salt
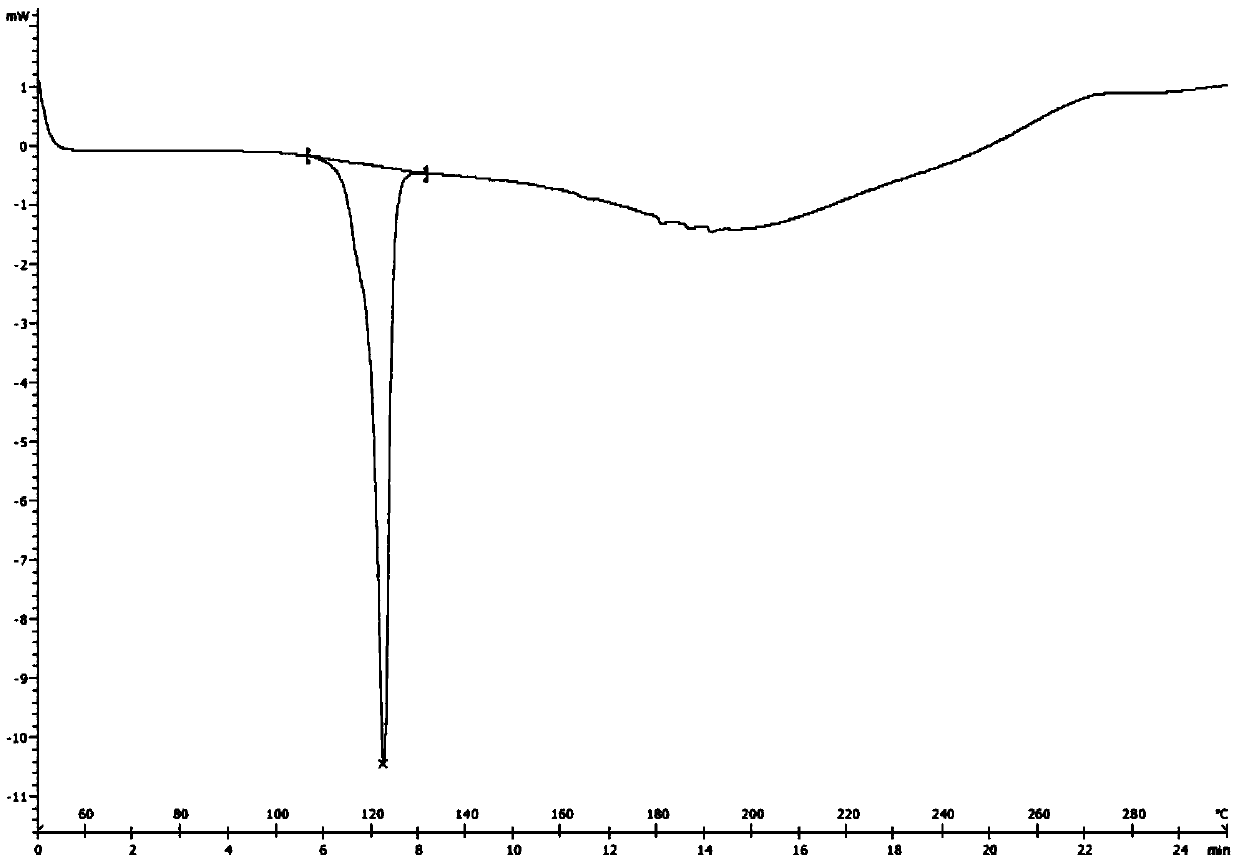
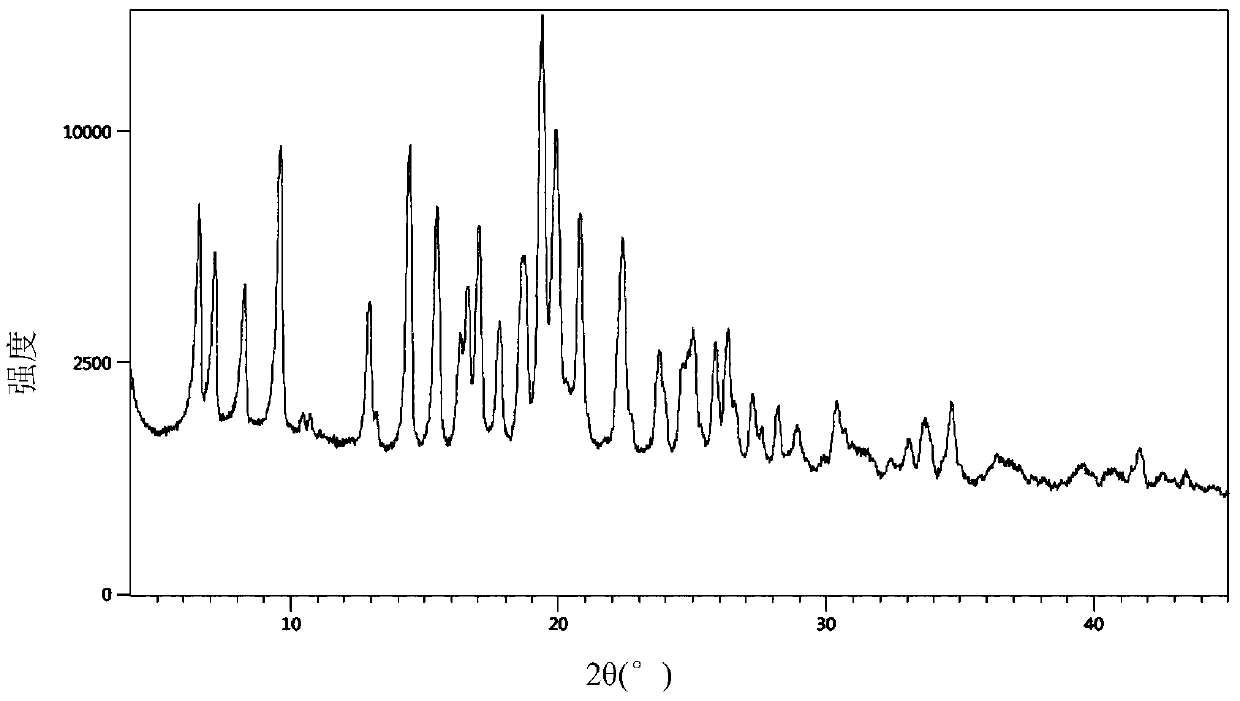
[0042] Add 6.0 g of the crude dicyclohexylamine salt of Sacubitril prepared in Example 1 into 50 mL of ethyl acetate, heat to 78°C to dissolve, stir for half an hour and then slowly cool to 2°C to crystallize, filter, and dry in vacuo Finally, 5.6 g of white crystals were obtained with a yield of 93% and a melting point of 122-123°C. The DSC figure of gained crystal (crystalline form I) sees figure 1 , powder X-ray diffraction pattern see figure 2 .
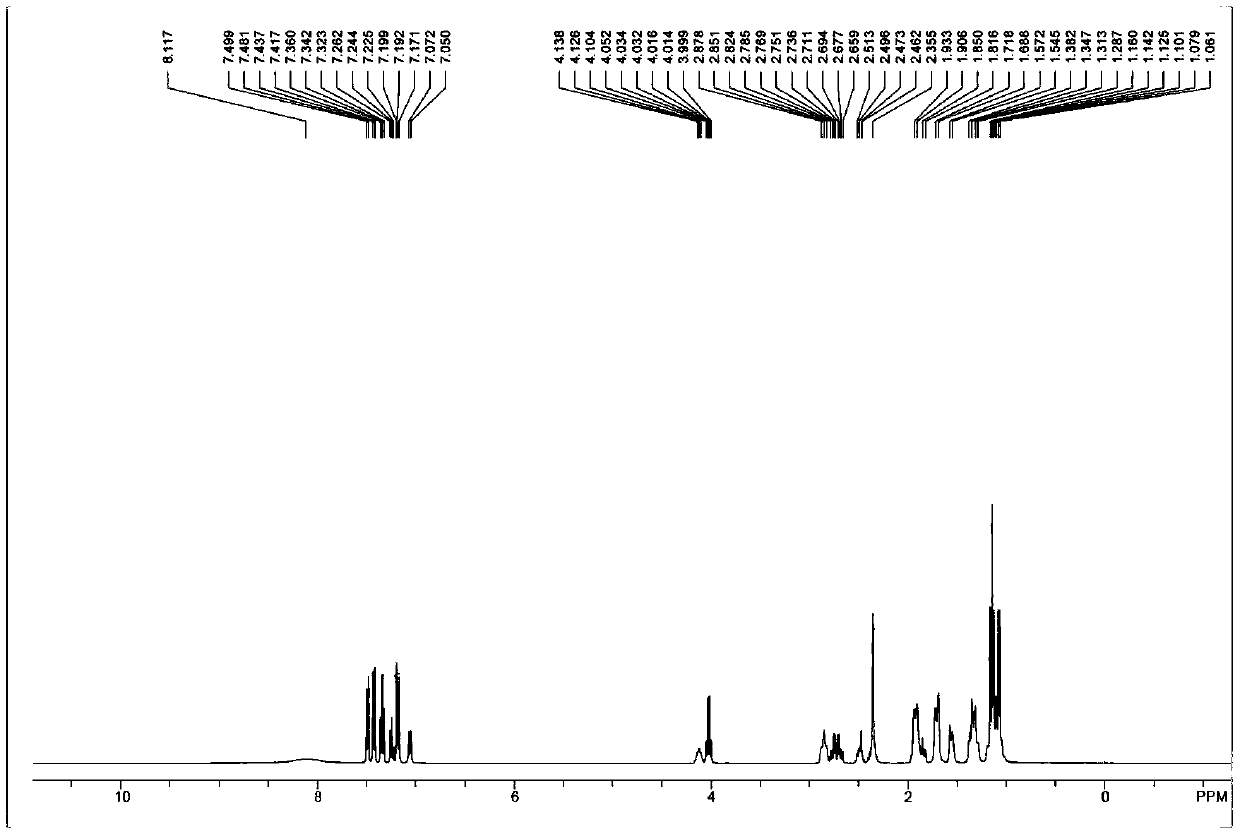
[0043] Sacubitril Dicyclohexylamine Salt 1 HNMR picture as shown image 3 Shown; Shakubi tricyclohexylamine salt after heavy water exchange 1 HNMR picture as shown Figure 4 Shown; Sacubitril dicyclohexylamine salt 13 CNMR picture as Figure 5 shown.
[0044] The NMR data are as follows: 1 HNMR (400MHz, CDCl 3 ):δ8.70(br,2H),7.49(m,2H),7.41(m,2H),7.37(m,1H),7.25-7.10(m,2H),4.05(br,1H),4.01( m,2H),2.86(m,2H),2.70(m,2H),2....
Embodiment 3~8
[0048] According to the operation process of Example 2, different solvents, dosages and temperature conditions were changed to carry out crystallization, the results are shown in the following list of examples, and the DSC figure of the obtained crystal form is shown in figure 1 , powder X-ray diffraction pattern see figure 2 , nuclear magnetic data is the same as embodiment 2, and HPLC collection of illustrative plates is as Figure 6 shown.
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com