Polycation quaternary ammonium salt high polymer material and preparation method thereof
A polymer material and polycation technology, applied in the field of polycation quaternary ammonium salt polymer material and its preparation, can solve problems such as undiscovered, and achieve the effects of stable performance, novel structure and easy processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] HCl 3 Preparation of Ox monomer:
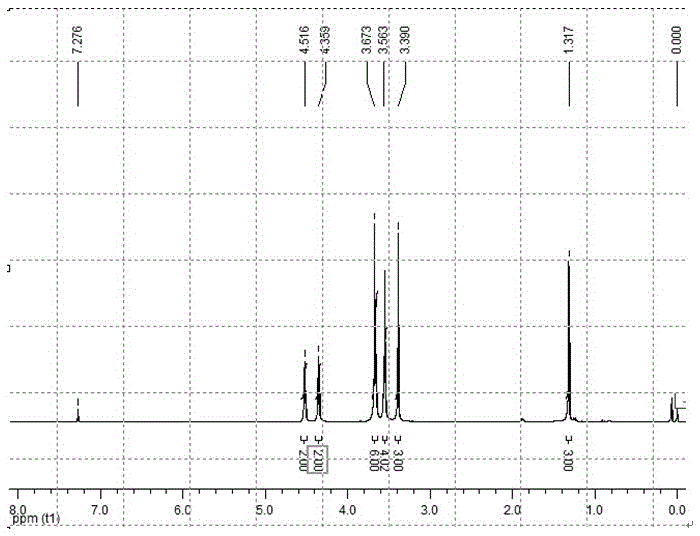
[0055] Stir 1,3-dichloropropane (10.5g, 93mmol), 3-hydroxymethyloxetane (2.7g, 31mmol), sodium hydroxide (20g, 500mmol) in a two-phase system of n-hexane and water Homogenously, use tetrabutylammonium bromide as a phase transfer catalyst, and react under reflux conditions for 0.5 hours. After the reaction was completed, it was purified by extraction and vacuum distillation to obtain a colorless liquid. Yield: 55%. 1 HNMR (300MHz, CDCl 3 ): δ1.74 (m, 2H, -CH 2 -CH 2 -CH 2 -Cl), δ3.01 (m, 1H, -CH-), δ3.36 (m, 6H, -CH 2 -O-CH 2 -CH 2 -CH 2 -Cl), δ4.34-4.51 (dd, 4H, -CH on the four-membered ring 2 -O-CH 2 -).
[0056] HE 1 Preparation of Ox monomer:
[0057] Ethylene glycol monomethyl ether (12.9g, 170mmol) was reacted with sodium hydride (4g, 170mmol) in anhydrous tetrahydrofuran until no bubbles were generated, and then 3-(p-toluenesulfonate methyl)oxy Heterocyclobutane (41.2 g, 170 mmol) was added dropwise to the reactio...
Embodiment 2
[0063] MeBr 4 Preparation of Ox monomer:
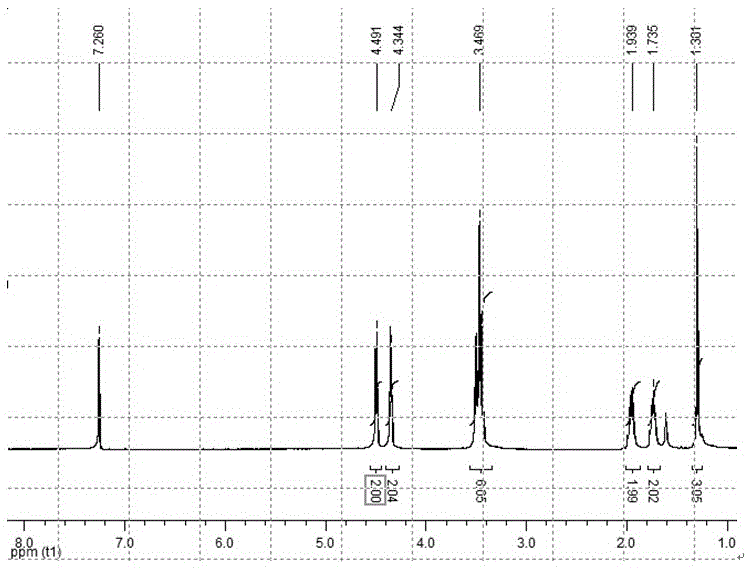
[0064] 1,4-dibromobutane (20g, 93mmol) and 3-methyl-3-hydroxymethyl oxetane (3.1g, 31mmol), sodium hydroxide (20g, 500mmol) in n-hexane and water Stir evenly in the two-phase system, use tetrabutylammonium bromide as a phase transfer catalyst, and react under reflux conditions for 0.5 hours. After the reaction was completed, it was purified by extraction and vacuum distillation to obtain a colorless liquid. Yield: 80%. The NMR spectrum is shown in Fig. 1 HNMR (300MHz, CDCl 3 ): δ1.30 (s, 3H, -CH 3 ), δ1.73 (m, 2H, -CH 2 -CH 2 -Br), δ1.95 (m, 2H, -CH 2 -CH 2 -CH 2 -Br), δ3.47 (m, 6H, -CH 2 -O-CH 2 -CH 2 -CH 2 -CH 2 -Br), δ4.34-4.51 (dd, 4H, -CH on the four-membered ring 2 -O-CH 2 -).
[0065] MeE 2 Preparation of Ox monomer:
[0066] Diethylene glycol monomethyl ether (20g, 170mmol) was reacted with sodium hydride (4g, 170mmol) in anhydrous tetrahydrofuran until no bubbles were produced, and then 3-methyl-3-(p-tolue...
Embodiment 3
[0072] Et 6 Preparation of Ox monomer:
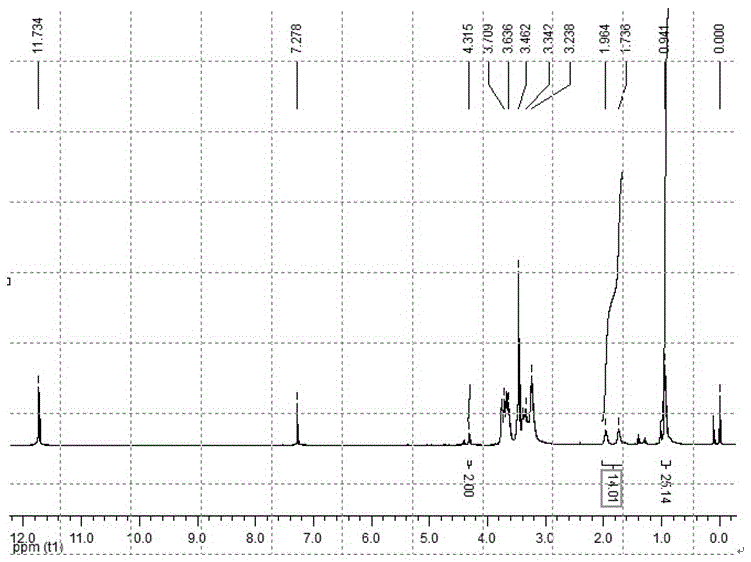
[0073] 1,6-diiodohexane (31.4g, 93mmol) and 3-ethyl-3-hydroxymethyl oxetane (3.6g, 31mmol), sodium hydroxide (20g, 500mmol) in n-hexane and Stir evenly in the water two-phase system, use tetrabutylammonium bromide as a phase transfer catalyst, and react under reflux for 0.5 hours. After the reaction was completed, it was purified by extraction and vacuum distillation to obtain a colorless liquid. Yield: 78%. 1 HNMR (300MHz, CDCl 3 ): δ0.90 (t,3H,-CH 2 -CH 3 ), δ1.20-1.50 (m, 6H, -CH 2 -CH 3 ,-CH 2 -CH 2 -CH 2 -CH 2 -I), δ1.72-1.95 (m, 4H, -O-CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -I), δ3.47 (m, 6H, -CH 2 -O-CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -CH 2 -I), δ4.34-4.51 (dd, 4H, -CH on the four-membered ring 2 -O-CH 2 -).
[0074] Et 4 Preparation of Ox monomer:
[0075]Tetraethylene glycol monomethyl ether (35.4g, 170mmol) was reacted with sodium hydride (4g, 170mmol) in anhydrous tetrahydrofuran until no bubbles were gener...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com