Arylsalicylaldehyde-diphenyl-azine hydrazine compound as well as preparation and application
A technology of aryl salicylaldehyde and diphenyl, which is applied in the field of analysis and detection materials, can solve problems such as difficult detection and fluorescence analysis, decrease in fluorescence quantum yield, high non-radiative transition ratio, etc., and achieve strong fluorescent probe characteristics , increase Stokes displacement, and enhance the effect of AIE performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of salicylaldehyde-diphenyl-azine hydrazine (DBAS), the synthetic route is shown in the following formula:
[0062]
[0063] The specific synthesis steps are: reflux reaction of 10g diphenylhydrazine and 5ml salicylaldehyde in 100ml ethanol solvent for 2 hours, cool to -10°C after the reaction is completed, filter at low temperature after a large amount of needle-packed solids are precipitated, and weigh the crude product with ethanol Crystallized twice to obtain pale colorless needle-packed crystals of salicylaldehyde-diphenyl-azinehydrazine (DBAS) with a purity of 99% and a yield of 90%. The product identification data are: 1 HNMR(400MHz,d-DMSO,ppm):11.28(s,1H,-OH),8.75(s,1H),7.64-7.62(m,2H),7.54-7.41(m,7H),7.28-7.24( m,3H), 6.29-6.26(m,1H), 5.95(d,1H). MALDI-TOF(m / z):[M+]calcd.C 20 h 14 N 2 O, 298.11; found, 298.11.
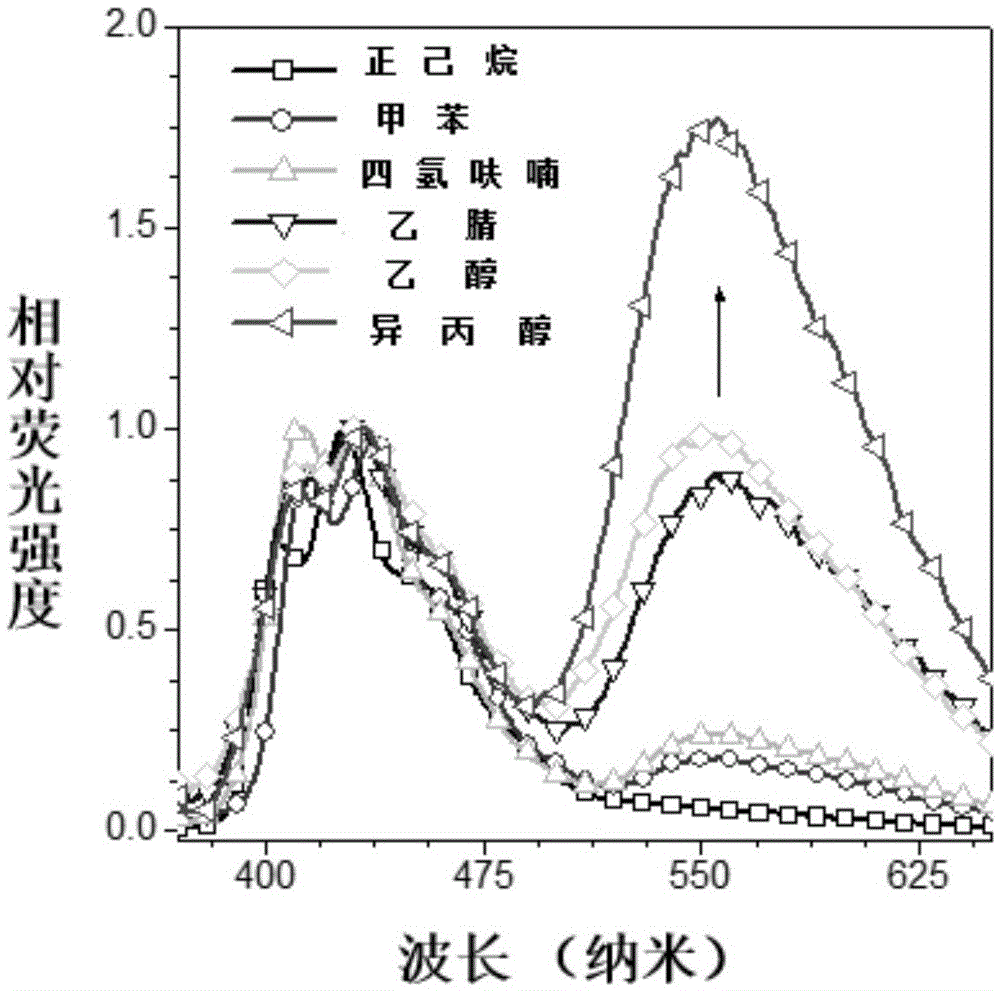
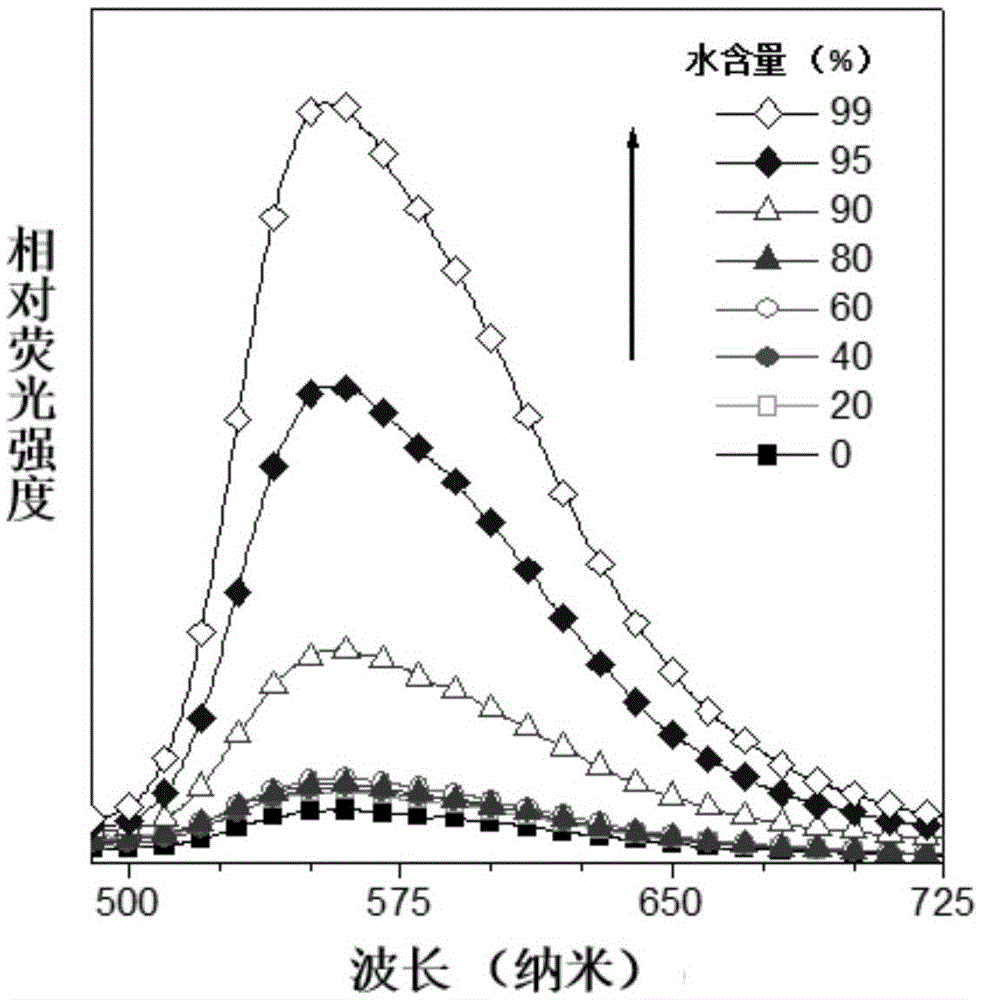
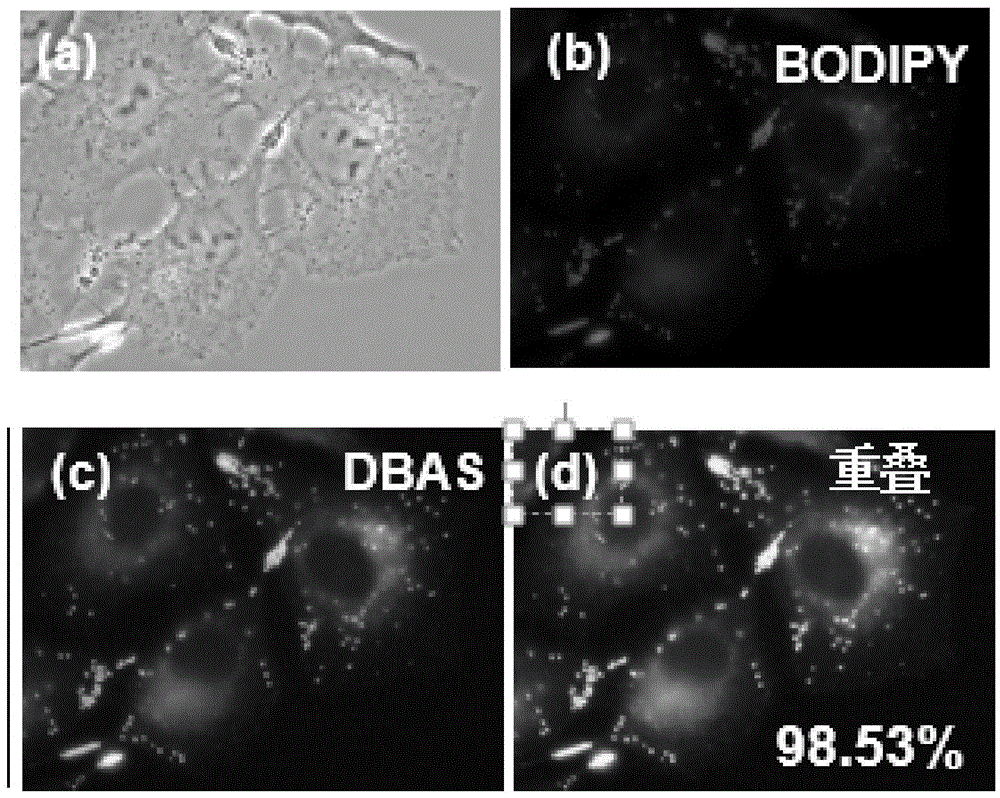
[0064] The optical properties of DBAS obtained in this example and its application in cell staining and metal ion sensing:
[0...
Embodiment 2
[0073] Take 1g of diphenylhydrazine crystals and equimolar 1-naphthyl salicylaldehyde in 50ml of ethanol and react at 60°C for 2h. After the reaction is completed, cool to obtain light yellow crystals. After filtering the solids, wash them with 75% aqueous ethanol. The obtained naphthyl salicylaldehyde-diphenyl-azine hydrazine (DBNAS) has a product purity of 99% and a yield of >95%. The product identification data is: MALDI-TOF(m / z):[M+]calcd.C 24 h 18 N 2 O, 350.41; found, 350.65. AnalCalc.forC 24 h 18 N 2 O: C, 82.26; H, 5.18; N, 7.99; O, 4.57. Found: C, 82.24; H, 5.16; N, 7.94; O, 4.60. The product structure is shown in the following formula:
[0074]
[0075] The optical properties of DBNAS obtained in this example and its application in intracellular staining:
[0076] (a) Basic optical properties of DBNAS:
[0077] The fluorescence spectra of DBNAS in different polar solvents are as follows: Figure 8 shown by Figure 8It can be seen that with the change of ...
Embodiment 3
[0081] The synthesis of DBAS nitrogen ethyl derivatives, concrete synthetic steps are as follows:
[0082] Take 1g of diphenylhydrazine (or N,N-diethylaminodiphenylhydrazine) and equimolar N,N-diethylaminosalicylaldehyde (or salicylaldehyde) in 30ml of ethanol at 60°C Reaction 4h, cooling after completion of the reaction, extraction and liquid separation, column chromatography to obtain the following corresponding products:
[0083]
[0084] N-DBAS: yellow solid, 87% yield. The product identification data is: MALDI-TOF(m / z):[M+]calcd.C 24 h 25 N 3 O: 371.47; found, 371.88; AnalCalc.forC 24 h 25 N 3 O: C, 77.60; H, 6.78; N, 11.31; O, 4.31. Found, C, 77.55; H, 6.74; N, 11.30; O, 4.32;
[0085] DBAS-2N: pale yellow solid, yield 85%. The product identification data is: MALDI-TOF(m / z):[M+]calcd.C 28 h 34 N 4 O: 442.27; found, 442.30; AnalCalc.forC 28 h 34 N 4 O: C, 75.98; H, 7.74; N, 12.66; O, 3.61. Found, C, 75.78; H, 7.69; N, 12.34; O, 3.71;
[0086] N-DBAS-2N: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com