Method using light on/off mode probe to measure solution pH
An optical switch and probe technology, applied in the field of analytical chemistry, can solve problems such as fluorescence intensity interference, and achieve the effect of expanding the application range and various measurement methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation method of each reagent in the method of the present invention.
[0044] (1) Probe solution: weigh 2.8 mg of probe (molecular formula: C 17 h 13 NO 3 Molecular weight: 279.09), dissolved in acetonitrile to prepare a 100mL solution with a concentration of 100μM.
[0045] (2) Tris-HCl buffer solution: prepared with a concentration of 50mM trishydroxymethylaminomethane (Tris) and 50mM HCl, and adjusted the pH to the desired value with a pH meter;
[0046] (3) HEPES-NaOH buffer solution: prepared with a concentration of 50mM 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) and 50mM NaOH, and adjusted the pH to the desired value with a pH meter;
[0047] (4) Other coexisting ions and molecular solutions: Take analytically pure nitrates or perchlorates of various metals, dissolve them in double-distilled water, and prepare a double-distilled aqueous solution with a concentration of 20mM; take analytically pure various Amino acids, bovine se...
Embodiment 2
[0049] Example 2: Preparation of probe compounds.
[0050] Using 8-hydroxyquinaldine and 2,4-dihydroxybenzaldehyde as raw materials, using acetic anhydride and pyridine / water as solvents, firstly synthesize the intermediate, and then hydrolyze the intermediate in a mixed solvent of pyridine / water. The synthetic route is as follows:
[0051]
[0052] In the there-necked flask, in the acetic anhydride solution that is dissolved with 8-hydroxyquinaldine, add 2,4-dihydroxybenzaldehyde, by molar ratio 8-hydroxyquinaldine:2,4-dihydroxybenzaldehyde equals 1: 2. Under the protection of nitrogen, reflux, the reaction is completed, concentrated to remove the solvent acetic anhydride, and eluted by silica gel column chromatography to obtain an intermediate. Reaction temperature: 139°C (reflux), reaction time: 5h, reaction solvent: acetic anhydride, eluent: volume ratio chloroform: ethyl acetate (3:1).
[0053] N 2 Under protection, add the intermediate in the three-necked flask, py...
Embodiment 3
[0054] Example 3: Spectral measurement of pH probes.
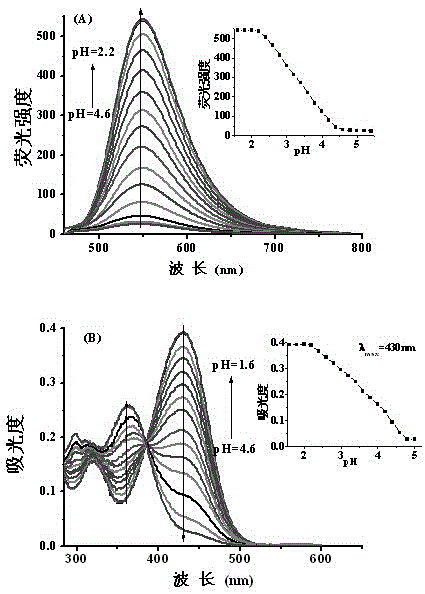
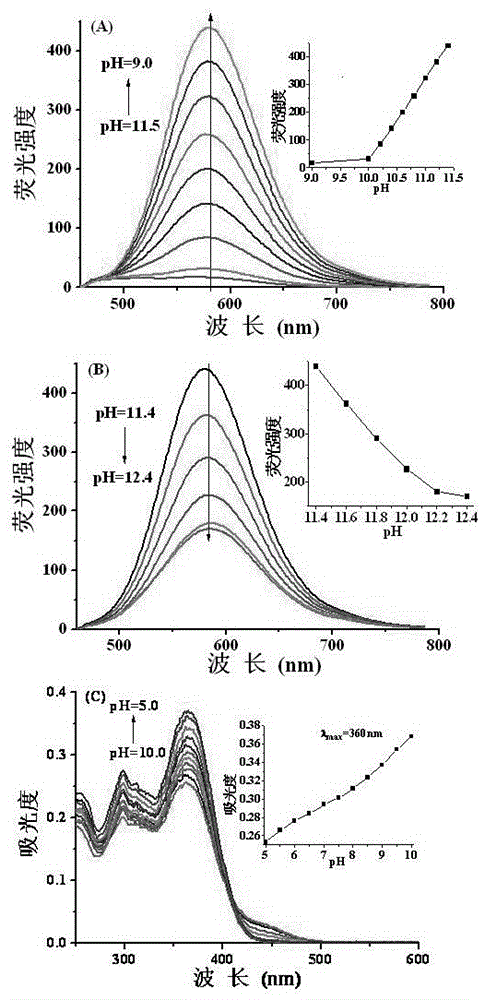
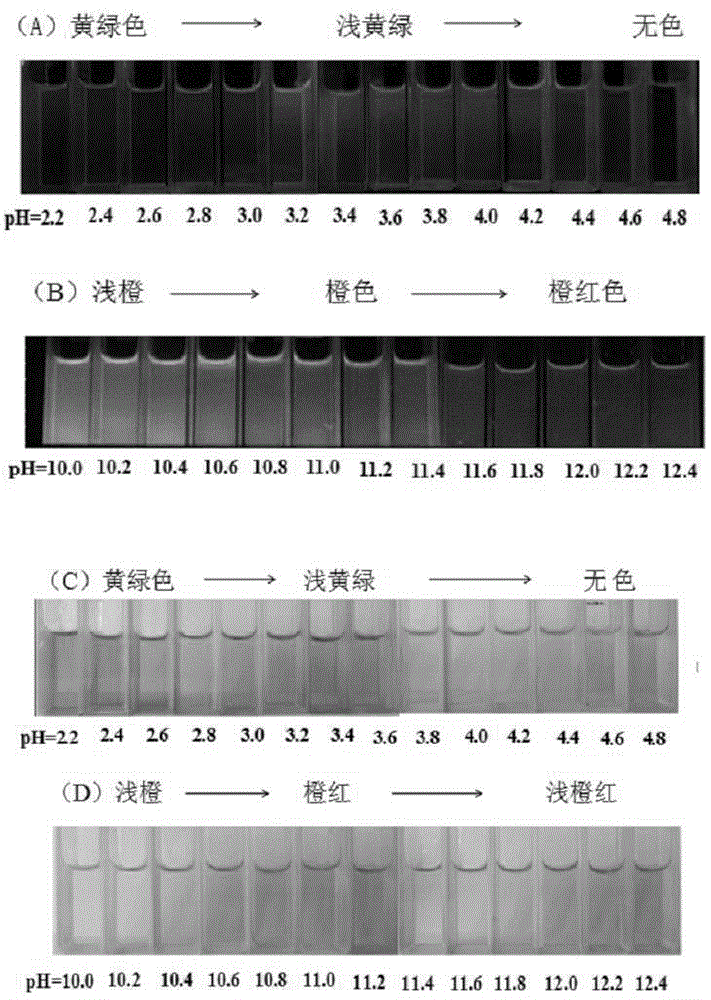
[0055] Add the acetonitrile stock solution (0.1mM, 1mL) of the probe into a 10.0mL volumetric flask, and add Tris-HCl or HEPES-NaOH buffer solutions with different pH values, so that the solvent ratio of the test solution is acetonitrile / buffer solution (v / v, 3 / 2), dilute to the mark, shake well, and transfer to a 1cm quartz cuvette for fluorescence spectrum and UV-visible absorption spectrum determination. The maximum excitation wavelength measured by fluorescence spectroscopy is 440nm, and the maximum emission wavelength is 550nm or 580nm, respectively.
[0056] (1) Detection of pH by fluorescence spectroscopy
[0057] In a 1cm cuvette, add the probe solution with a concentration of 10μM, and then add the probe solution with a pH of 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, 3.0, 3.2, 3.4, 3.6, 3.8, 4.0, 4.2, 4.4, 4.6, 4.8, 5.0, 5.2, 5.4 Tris-HCl buffer solution, so that the solvent ratio of the test solution is (acetonitrile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com