Organic multi-pore supported catalyst as well as synthesis method and application thereof
A supported catalyst, hierarchical pore technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of backward research, lack of synthesis mechanism and preparation method, etc. , to achieve the effect of good universality, excellent multi-channel connectivity, and controllable size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1 The synthesis of the precursor of organic hierarchical porous loaded organic amine catalyst
[0064] (a) Synthesis of PGM main chain:
[0065] Glycidyl methacrylate (2ml), azobisisobutyronitrile (AIBN, 2.4mg), RAFT reagent (36mg), and benzene (2ml) were added to the reaction test tube, and the reaction was sealed at 60°C after removing oxygen. After the reaction, it was precipitated in methanol. Dichloromethane dissolved. NMR results showed that its degree of polymerization was 250.
[0066] (b) Hydrolysis of the PGM backbone:
[0067] The main chain (1 g) synthesized in step 1 was dissolved in tetrahydrofuran (THF, 20 ml), glacial acetic acid (40 ml), and 60 ml of water was slowly added at 60°C. Reacted for 24h, and precipitated in ether after the reaction. dissolved in methanol.
[0068] (c) Synthesis of P(GM-g-LA):
[0069] Dissolve 50mg of hydrolyzed PGM in 5ml of dry N,N-dimethylformamide, add 1620mg of recrystallized D,L-lactide, and then add 4...
Embodiment 2
[0076] Synthesis of embodiment 2 organic hierarchical porous supported organic amine catalyst
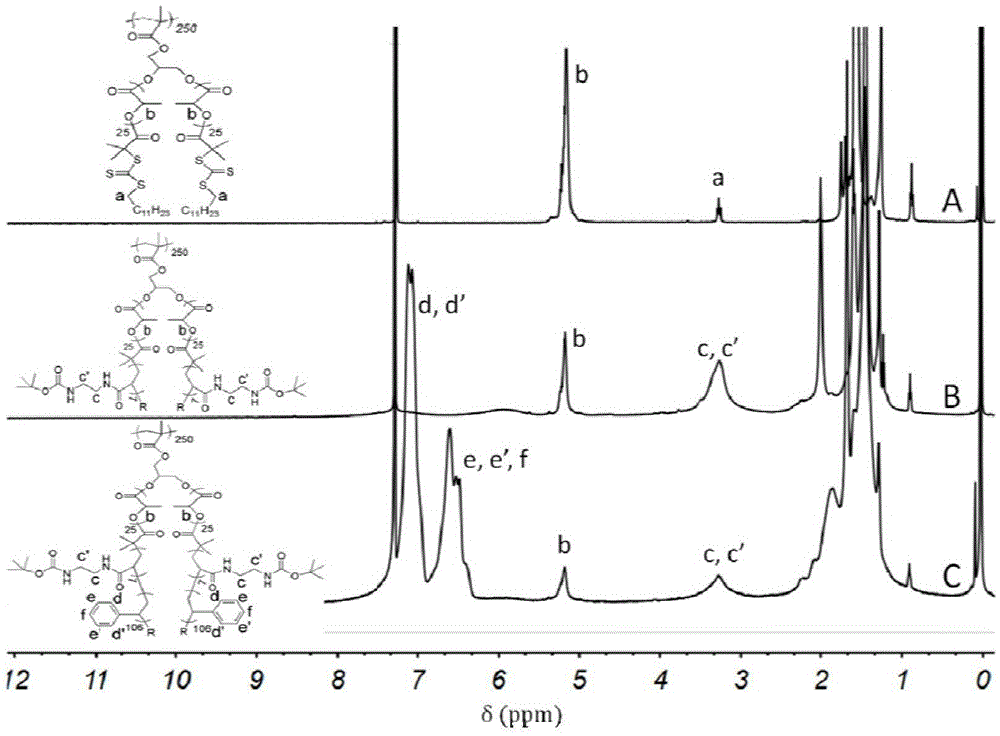
[0077] In a round-bottomed flask, 70 mg of P(GM-g-LA-g-TBOCa-g-St) prepared in Example 1 was dissolved in dry 7 ml of 1,2-dichloroethane, and after deoxygenation, 133 uL ( 1.5mmol) of dimethyl formal and 243mg (6mmol) of ferric chloride were reacted at 80°C for 14h. After the end, the obtained insoluble solid was washed with water and methanol respectively until the supernatant was colorless. Disperse the insoluble solid in 6ml 1,4-dioxane, add 0.6ml HCl solution, and react at room temperature for 12h. After the end, the insoluble solids were washed with water and methanol respectively until the supernatant was neutral. Dry in vacuum at 50°C for 24h. Figure 5 Infrared schematic diagram showing the process of cross-linking hydrolysis of organic hierarchical porous supported catalyst, compared with (A), 1758cm in (C) -1 The carbonyl characteristic peak of polylactic acid disappear...
Embodiment 3
[0078] Example 3 Catalysts of organic hierarchically porous loaded organic amines (amino groups) and organic small molecule catalysts (n-butylamine) catalyze the reaction of benzaldehyde and ethyl cyanoacetate in catalytic toluene solution
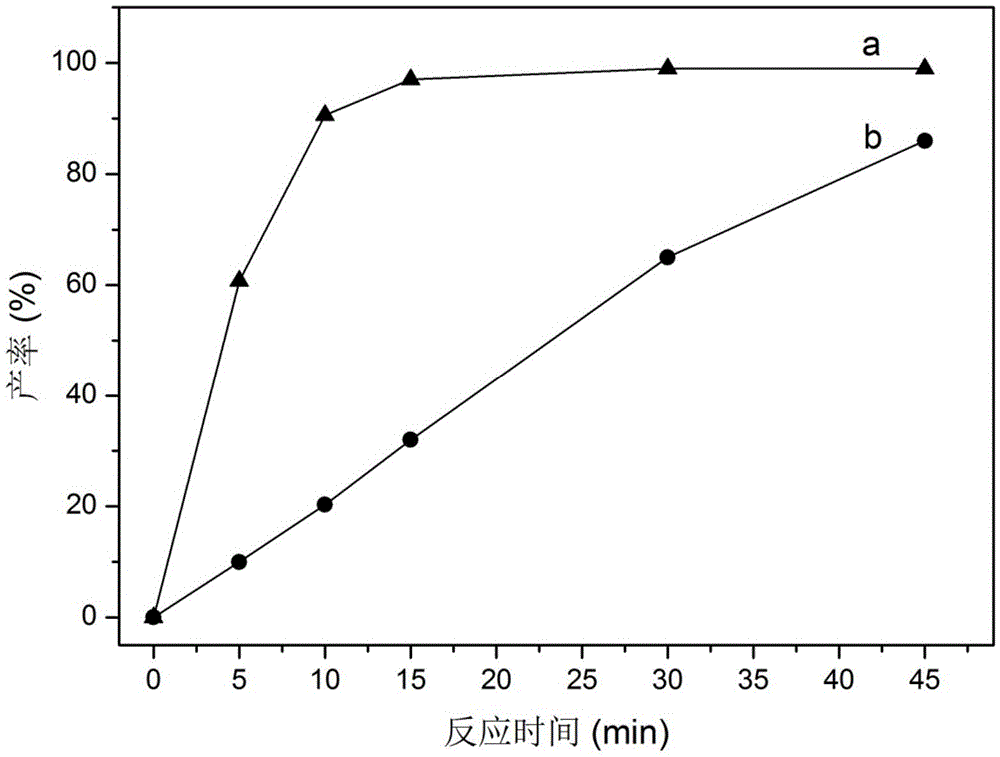
[0079] In a reaction flask, 5 mg of the organic hierarchically porous supported catalyst loaded with amino groups was dispersed and dissolved in 2.3 ml of toluene, and 17.5 ul (0.17 mmol) of benzaldehyde and 74 ul (0.68 mmol) of ethyl cyanoacetate were added. React at 80°C for 45min. The reaction solution was obtained by centrifugation, and the GC-MS result showed that the conversion rate of benzaldehyde was 97% after 15min, as figure 1 as shown in a.
[0080] Described reaction is shown in following reaction formula (II):
[0081]
[0082] Benzaldehyde (0.17mmol), ethyl cyanoacetate (0.68mmol) and organic hierarchically porous supported catalyst (30mg) were reacted in toluene (2.3ml) at 80°C for 45min.
[0083] In a reaction flask,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com