A kind of pentaptycene conjugated polymer and its preparation method and its application in rapid detection of nitroaromatic explosives
A technology of conjugated polymers and nitroaromatic hydrocarbons, applied in the field of polymer materials, can solve the problems such as sensitivity and selectivity to be improved, and achieve the effects of good selectivity and sensitivity, high sensitivity, high selectivity and rapid identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
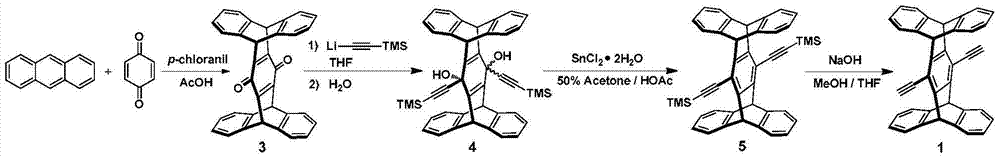
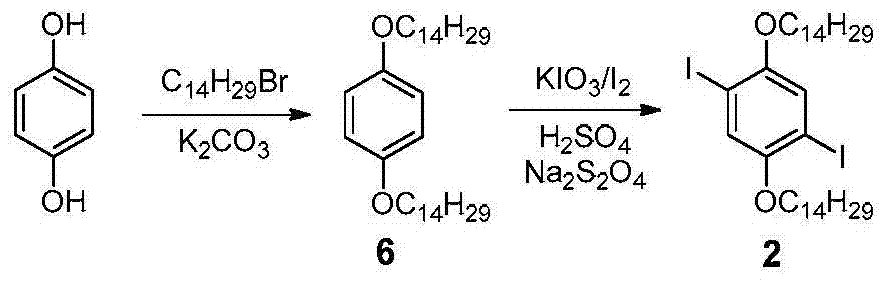
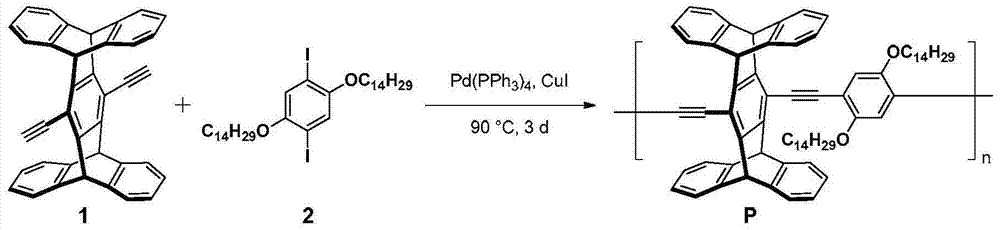
[0053] Embodiment 1, the synthesis of conjugated polymer P
[0054] according to image 3 The synthetic route diagram of conjugated polymer P is shown for the synthesis of conjugated polymer P. The specific operation is as follows:
[0055] Under argon protection, weigh compound 1 (40mg, 0.084mmol), 2 (62mg, 0.084mmol), Pd (PPh 3 ) 4 (5mg, 0.004mmol) and CuI (6mg, 0.03mmol) were placed in a 25mL Schlenk bottle, and 5mL of a dry mixed solvent of diisopropylamine and toluene 2:3 was added as a solvent, and reacted at 75°C for 3 days. After the reaction was completed, it was cooled to room temperature, spin-dried, 30 mL of methanol was added to the residue for precipitation, and it was filtered by suction to obtain 80 mg of P as a bright yellow powder with a yield of 95.2%.
[0056] The structure detection result of this compound is as follows:
[0057] 1 H NMR (300MHz, CDCl 3 )( Figure 4 is the H NMR spectrum of the conjugated polymer P): δ7.50–7.39(m,10H),7.03(brs,8H),...
Embodiment 2
[0060] Embodiment 2, the measurement of the ultraviolet-visible absorption spectrum and fluorescence spectrum of prepared conjugated polymer P
[0061] Dissolve the conjugated polymers prepared in Example 1 in chloroform respectively to configure a concentration of 1.0×10 -6 mol / L solution, and then carry out the determination of ultraviolet-visible absorption spectrum and fluorescence spectrum, and the corresponding test results are shown in Table 1.
[0062] Figure 5 It is the ultraviolet absorption spectrum of the conjugated polymer P prepared in Example 1.
[0063] Image 6 It is the fluorescence emission spectrum of the conjugated polymer P prepared in Example 1.
[0064] Table 1 is the measurement result of the ultraviolet-visible absorption spectrum and fluorescence spectrum of conjugated polymer P
[0065]
Embodiment 3
[0066] Embodiment 3, the mensuration of the thermal stability of the prepared conjugated polymer P
[0067] P was dissolved in chloroform to form a 1 mg / mL solution, and then spin-coated on a quartz wafer (10 mm × 10 mm) on a spin coater at a speed of 3000 rpm to form a thin film. Then the fluorescence spectra were measured at different temperatures. The result is as Figure 7 shown.
[0068] Figure 7 It is the fluorescence curve of P thin film at different temperatures.
[0069] Depend on Figure 7 It can be seen that as the temperature rises, the fluorescence intensity of the film increases slightly and then slowly decays. When the temperature reaches 50 degrees, the fluorescence intensity of the film increases by 20%. After continuing to increase the temperature to 70 degrees, the fluorescence intensity decreases slightly to the room temperature intensity. stabilized thereafter. This result demonstrates that the film is stable to temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com