Preparation method of novel nasal administration brain-targeted nano drug loading system
A nano-drug-loading, brain-targeting technology, applied in the field of medicine, can solve the problem that drugs cannot enter the brain tissue, and achieve the effects of improving brain targeting, good bioadsorption, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Recoilamine Nasally Administered Nano-brain Targeted Drug
[0032] This example provides a preparation method of the nanobrain-targeted drug for intranasal administration of Licolamine of the present invention, which specifically includes the following steps:
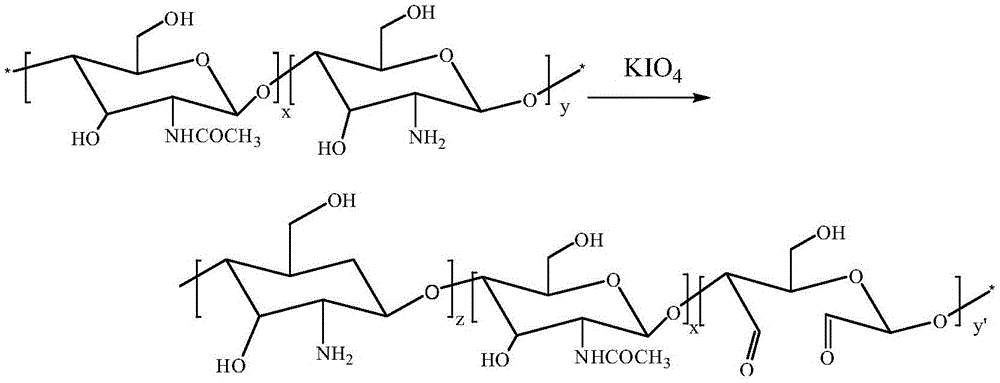
[0033] 1) Under nitrogen atmosphere, 10 mol chitosan acetic acid-sodium acetate buffer solution and 1 mol potassium periodate were stirred and reacted at 4°C for 48 hours, then 1 mol ethylene glycol solution was added to the solution to stop the reaction, and the product was concentrated by rotary evaporation Dialyzed in 1mol / L NaCl aqueous solution and deionized water for 48h to remove impurities, and the final product was freeze-dried to obtain formaldehyde chitosan (CS-CHO);
[0034] 2) Dissolve 1 mol of formylated chitosan (CS-CHO) in 3 mol of DMSO, then add 1 mol of sodium chlorite, and stir for 24 hours. Impurities, the final product is freeze-dried to obtain ortho-dicarboxylated chitosan (CS-COO...
Embodiment 2
[0036] The in vitro drug release research of embodiment 2 chitosan-loaded diuretics
[0037] The drug is placed in a dialysis bag, and samples are taken from the release medium at regular intervals to analyze the drug content. The specific process is to freeze-dry the obtained drug solution, accurately weigh a certain amount, re-disperse it in 10ml of artificial cerebrospinal fluid by ultrasonic, put it into a dialysis bag (MWCO=12,000) and place it in a 100ml Erlenmeyer flask, add 40ml of artificial cerebrospinal fluid at a constant temperature In the shaker (setting temperature 37°C, rotation speed 60r / min), sample 2ml at intervals, and supplement the same amount of release medium. This method can ensure that the polymer micelles remain in the dialysis bag, making the determination of the drug content more accurate.
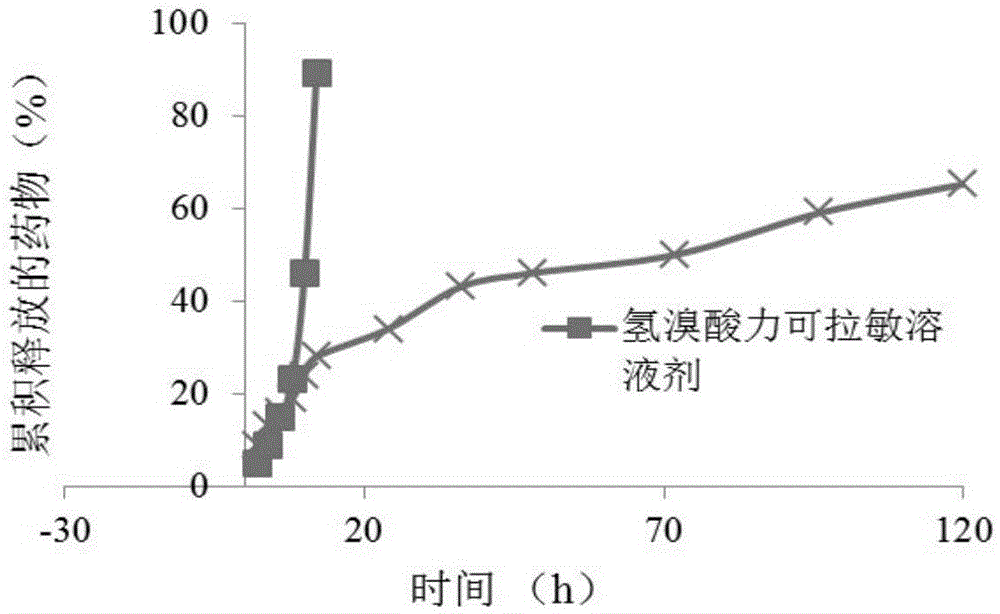
[0038] The result is as figure 1As shown, the release rate of Ricosylamine hydrobromide solution is relatively fast, and it is basically completely released ...
Embodiment 3
[0039] Example 3 Evaluation of Brain Targeting
[0040] Get healthy rats, be divided into 4 groups at random, be respectively Licolamine original drug group (original drug group); Nano drug-loaded micelles group (experimental group); Blank carrier micelles (blank group); control group). No food or water was allowed 24 hours before the experiment.
[0041] Rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium solution (40m / kg), fixed on the rat board in a supine position, cut the neck skin and muscles, separated the trachea, esophagus, esophagus, and trachea, and performed tracheal intubation, esophagus The upper segment is ligated to prevent the loss of drug solution to the gastrointestinal tract after nasal administration. During the whole experiment, the anesthesia was supplemented timely to ensure that the rats were always under anesthesia.
[0042] 30 minutes after the rat operation, the nasal administration group was administered with a micro-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com