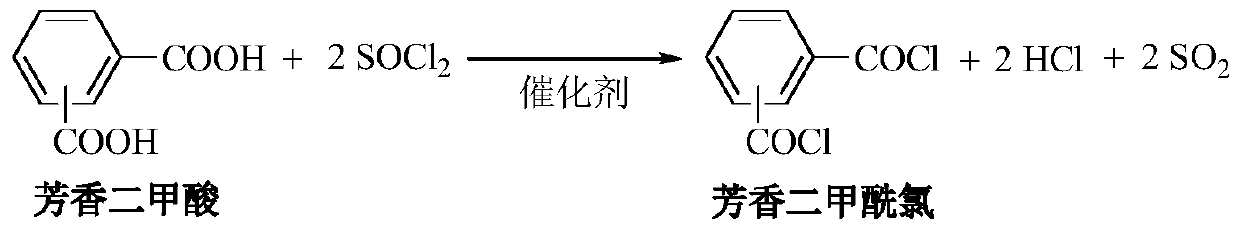
A kind of method for preparing aromatic diacid chloride
An aromatic diacid chloride and aromatic technology, which is applied in the field of preparation of aromatic diacid chloride, can solve the problems of long reaction time, shortened reaction time, and low product yield, and achieves short reaction time, simple and convenient reaction operation, and high product yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 5.0 grams of terephthalic acid (PTA), 20.0 grams of thionyl chloride (SOCl 2 ) and 0.1 gram of 1-methylpyrrolidine were added into a 50ml reactor equipped with a reflux condenser, heated to reflux at 130°C for 2 hours, the generated HCl and SO 2 The gas is passed into water and 30% NaOH solution sequentially in time, and the pressure in the reactor is 0.1MPa. Recovery of catalyst and excess SOCl by distillation 2 , Distilled under reduced pressure to obtain 1.8 grams of product terephthaloyl chloride (TPC), the molar yield relative to PTA was 36%. The product was detected by GC-MS and 1 H NMR, 13 CNMR is used for qualitative analysis, and the purity detected by gas chromatography and liquid chromatography is higher than 99%.
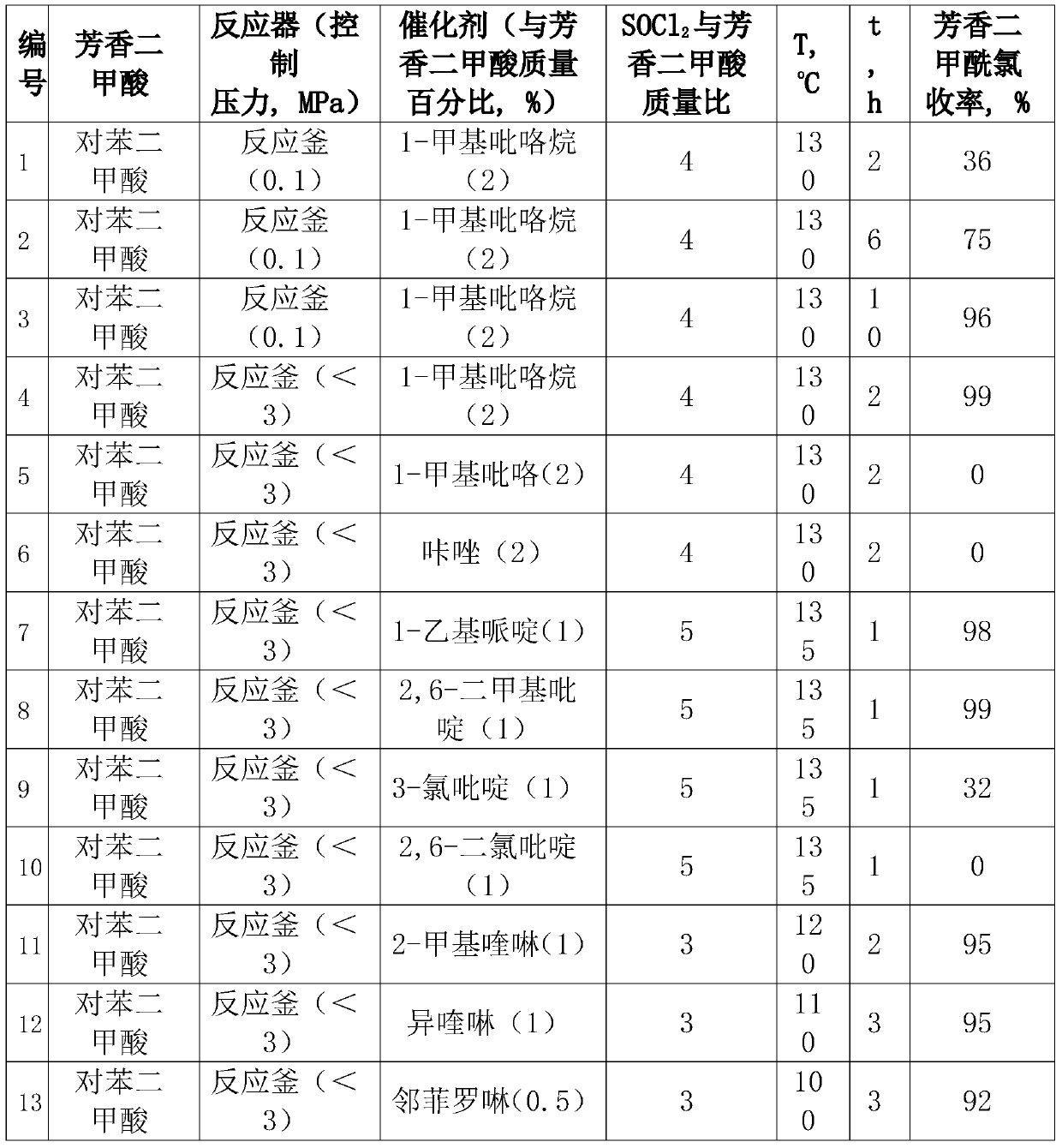
[0018] Table 1. Aromatic dicarboxylic acid and SOCl 2 Reaction Conditions and Results of Preparation of Aromatic Diformyl Chloride
[0019]
[0020]
Embodiment 2~3
[0022] The specific procedure of Examples 2-3 is similar to that of Example 1, and the specific reaction conditions and results are shown in Table 1.
Embodiment 4
[0024] 5.0 grams of PTA, 20.0 grams of SOCl 2 Add 0.1 gram of 1-methylpyrrolidine into a 50ml reactor, heat at 130°C for 2 hours, the pressure in the reactor gradually rises to 2.8MPa, cool down to 25°C, and the gas in the reactor is sequentially passed into water and 30% NaOH solution , the HCl and SO generated by the absorption reaction 2 gas. After the reaction, the mixture is distilled to recover the catalyst and excess SOCl 2 , and then distilled under reduced pressure to obtain 6.0 g of product TPC with a molar yield of 99%. The product was detected by GC-MS and 1 H NMR, 13 C NMR for qualitative analysis, gas chromatography, liquid chromatography detection purity higher than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com