Genetic engineering subunit mixed vaccine as well as preparation method and application thereof
A genetic engineering and mixed vaccine technology, applied in the field of veterinary vaccine research, can solve the problems of incomplete cleavage, strong virulence, and decreased immunogenicity of the vaccine, and achieve the effect of high expression product yield and good antigenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The recombinant protein preparation of embodiment 1, APPApxIA, ApxⅡA and OMPD
[0063] 1. Main material
[0064] 1.1 The strain APP is a product of the China Veterinary Drug Administration, and the number is ActinobacilluspleuropneumoniaeCVCC259.
[0065] 1.2 Plasmids, vectors and competent cells
[0066] Escherichia coli (E.coli) BL21(DE3) and Escherichia coli (E.coli) DH5α competent cells were purchased from Takara Company, and expression vectors PET-32a(+) and PGEX-4T-1 were purchased from Novagen Company.
[0067] 2. Method
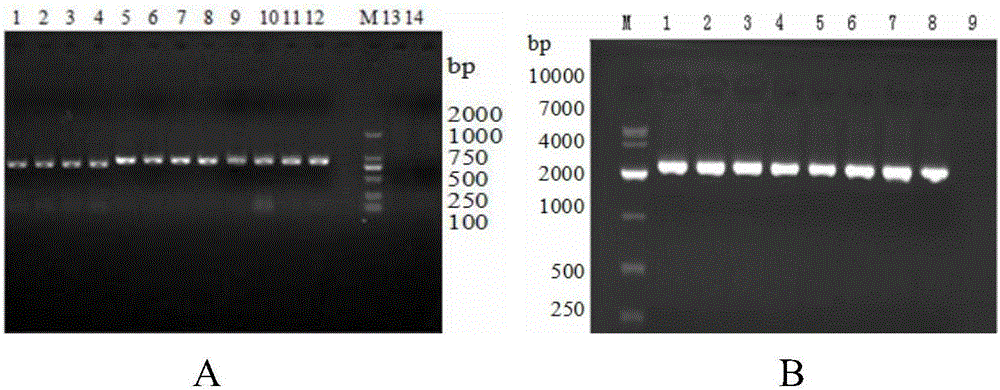
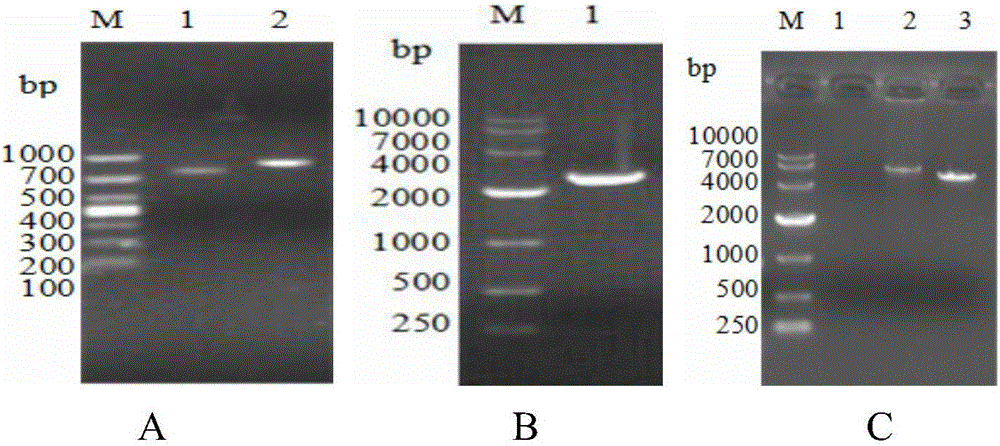
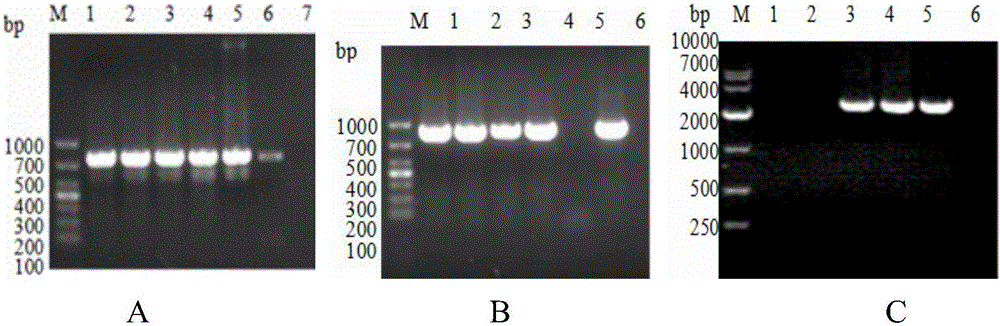
[0068] 2.1 Use the PCR method to expand the sequences in APPApxⅠA, ApxⅡA and OMPD genes SEQIDNO:1, SEQIDNO:2, and SEQIDNO:3, and the amplified lengths are 753bp, 843bp, and 2382bp respectively. The results are as follows figure 1 shown. The upstream and downstream primers F1, F2, F3, F4, F5, and F6 used were the aforementioned primers, all of which contained BamHI and XhoI restriction sites. PCR reaction system: 2×KODFXBuffer 25 μL, 2mMdN...
Embodiment 2
[0116] Example 2 Analysis of Immune Efficacy of Porcine Infectious Actinobacillus Pleuropneumoniae ApxⅠA, ApxⅡA and OMPD Genetic Engineering Subunit Mixed Vaccine
[0117] 1. Main material
[0118] 1.1 Animals: 4-6 weeks old female Balb / c mice, purchased from Guangzhou Southern Medical University Experimental Animal Technology Co., Ltd.
[0119] 1.2 Vaccine: It is the purified protein obtained in Example 1 and a mixture of three kinds of proteins in equal mass. The protein is emulsified with Freund's complete adjuvant and Freund's incomplete adjuvant, both of which are purchased from SIGMA.
[0120] 2. Method
[0121] 2.1 Take 60 Balb / c mice aged about 4 to 6 weeks and divide them into 6 groups at random, rApxIA, rApxIIA, rOMPD and rApxIA+rApxIIA+rOMPD proteins are each in one group, and normal saline is used as the blank control group and pigs infected with The trivalent inactivated vaccine of acute pleuropneumonia (purchased from Wuhan Keqian Company) was used as a positiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com