Rate hydrogen sulfide chemical dosimeter and preparation method and application thereof
A technology of hydrogen sulfide chemistry and dosimetry, applied in the field of detection, can solve problems such as the decrease in the accuracy of analysis results, and achieve the effects of high yield, accurate detection and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 0.235g of 1,2-lutidine iodide and 0.310g of 4-nitrobenzaldehyde into a 50ml round bottom flask, add 20ml of n-butanol, add 0.01g of piperazine, heat the mixed solution to reflux, and react for 40min . After the reaction was detected by TLC, it was cooled to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and n-butanol was recrystallized to obtain 0.230 g of an orange-red solid. The yield was 62.5%.
[0032] Melting point determination: mp: 239-242°C
[0033] Infrared spectrometry: TR (KBr, cm -1 )3346, 2997, 1635, 1616, 1567, 1516, 1457, 1349
[0034] H NMR spectrum determination: 1 HNMR (400MHZ, DMSO): δ (ppm): 9.02 (d, J = 6.0Hz, 1H), 8.60 (m, 2H), 8.36 (d, J = 8.8Hz, 2H), 8.16 (d, J = 8.8 Hz, 2H), 8.08(d, J=16Hz, 1H), 8.03(m, 1H), 7.86(d, J=16Hz, 1H), 4.45(s, 3H).
Embodiment 2
[0036] Weigh 0.2350g of 1,2-lutidine iodide and 0.3875g of 4-nitrobenzaldehyde into a 50ml round bottom flask, add 25ml of n-butanol, add 0.025g of piperazine, heat the mixed solution to reflux, and react for 30min . After the reaction was detected by TLC, it was cooled to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and n-butanol was recrystallized to obtain 0.242 g of an orange-red solid. The yield was 65.8%.
[0037] Melting point determination: mp: 239-242°C
[0038] Infrared spectrometry: TR (KBr, cm -1 )3346, 2997, 1635, 1616, 1567, 1516, 1457, 1349
[0039] H NMR spectrum determination: 1 HNMR (400MHZ, DMSO): δ (ppm): 9.02 (d, J = 6.0Hz, 1H), 8.60 (m, 2H), 8.36 (d, J = 8.8Hz, 2H), 8.16 (d, J = 8.8 Hz, 2H), 8.08(d, J=16Hz, 1H), 8.03(m, 1H), 7.86(d, J=16Hz, 1H), 4.45(s, 3H).
Embodiment 3
[0041] Weigh 0.2350g of 1,2-lutidine iodide and 0.355g of 4-nitrobenzaldehyde into a 50ml round bottom flask, add 22ml of n-butanol, add 0.018g of piperazine, heat the mixed solution to reflux, and react for 30min . After the reaction was detected by TLC, it was cooled to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and n-butanol was recrystallized to obtain 0.235 g of an orange-red solid. The yield was 63.9%.
[0042] Melting point determination: mp: 239-242°C
[0043] Infrared spectrometry: TR (KBr, cm -1 )3346, 2997, 1635, 1616, 1567, 1516, 1457, 1349
[0044] H NMR spectrum determination: 1 HNMR (400MHZ, DMSO): δ (ppm): 9.02 (d, J = 6.0Hz, 1H), 8.60 (m, 2H), 8.36 (d, J = 8.8Hz, 2H), 8.16 (d, J = 8.8 Hz, 2H), 8.08(d, J=16Hz, 1H), 8.03(m, 1H), 7.86(d, J=16Hz, 1H), 4.45(s, 3H).
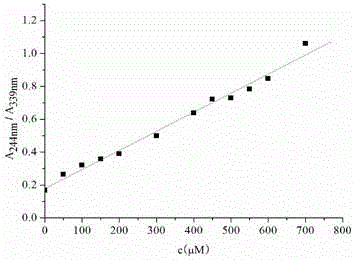
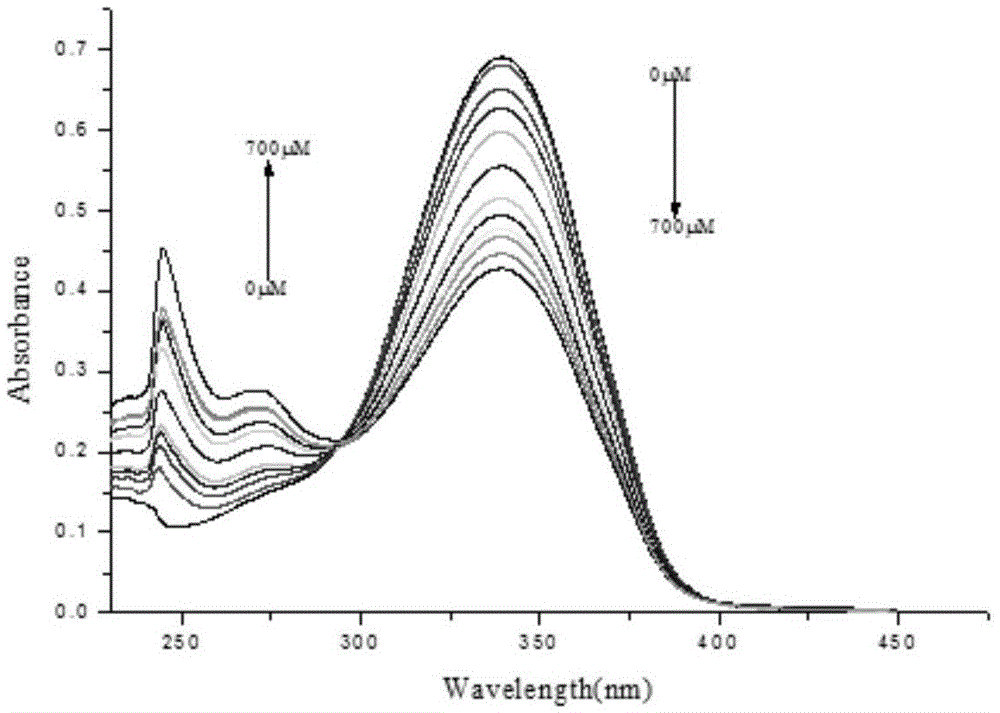
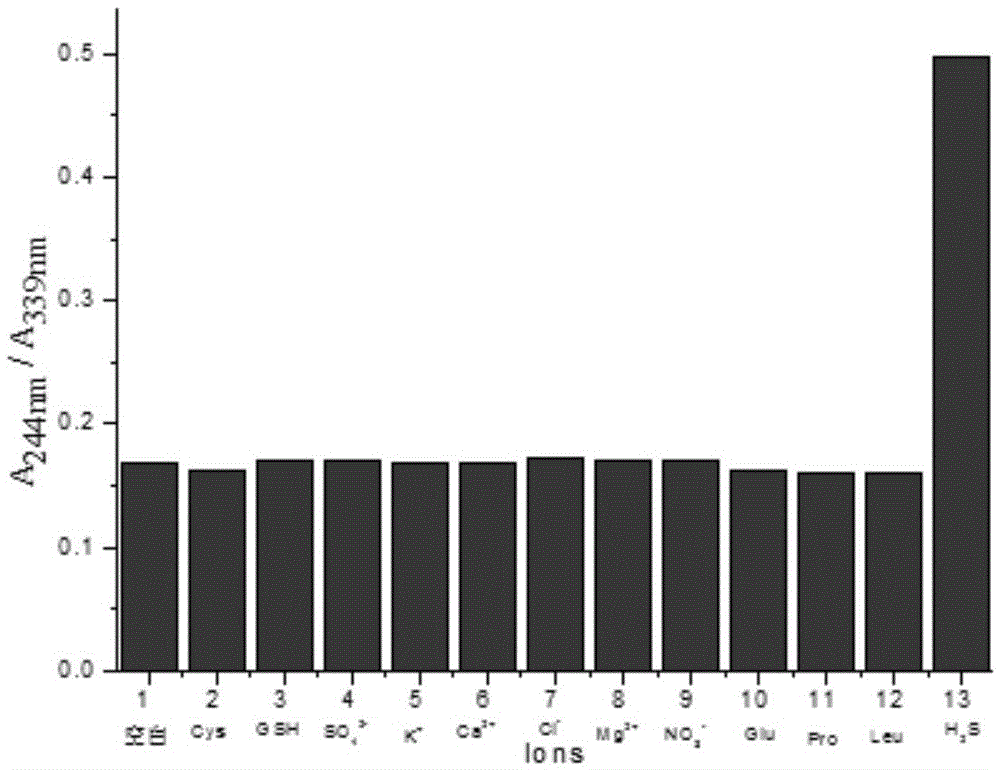
[0045] Effect experiment 1
[0046] Get the ratio hydrogen sulfide chemical dosimeter obtained in Example 1 and dissolve it in the DMF solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com