1,3-1,4-beta-glucanase mutant
A technology of dextranase and mutants, which is applied in the fields of genetic engineering and enzyme engineering, can solve the problems that thermal stability cannot be adapted to industrial applications, and achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
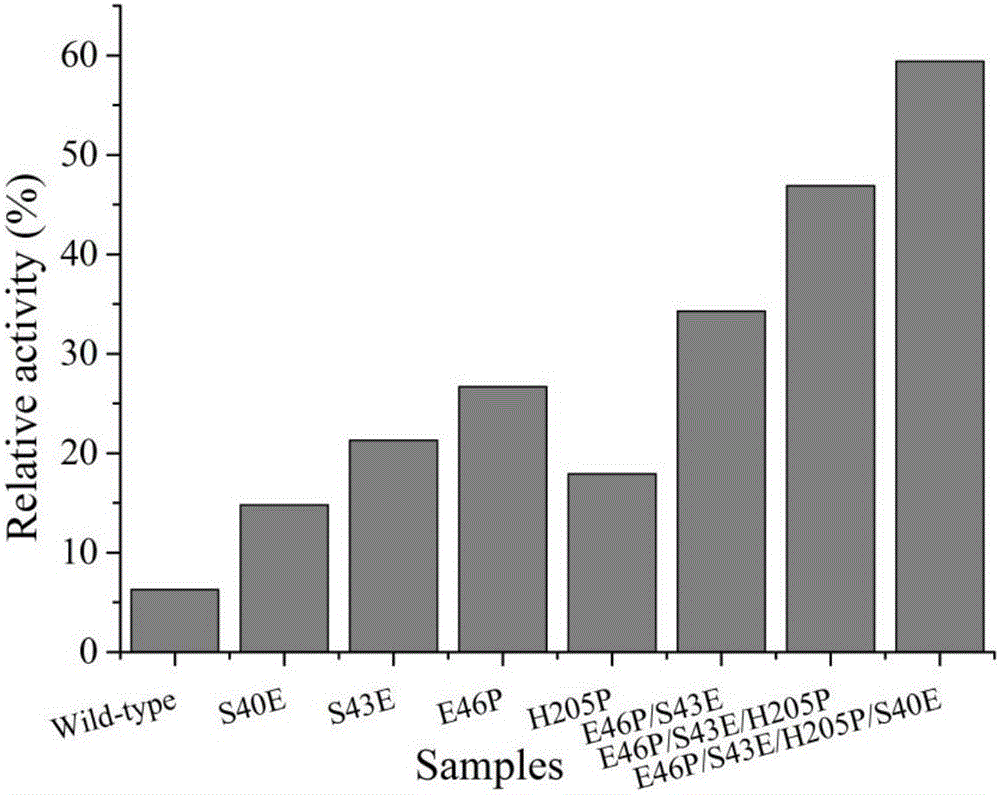
[0026] Iterative saturation mutation of embodiment 1β-glucanase
[0027] Iterative saturation mutation was carried out at four positions 40, 43, 46 and 205 and the thermostability parameters of the mutants were determined. Using the plasmid pET28a(+)-BglTM (NiuC, ZhuL, ZhuP, LiQ.2015.Lysine-BasedSite-DirectedMutagenesisIncreasedRigidβ-SheetStructureandThermostabilityofMesophilic1,3–1,4-β-Glucanase.JournalofAgriculturalandFoodChemistry63:5249-5256) as a template, The 43rd, 46th and 205th positions introduced NNK degenerate codons (N: Ade / Cyt / Gua / Thy; K: Gua / Thy) by the Quickchange method to replace the target amino acids.
[0028] The site-directed mutagenesis primers for introducing the NNK degenerate codon at position 40 are:
[0029] Forward primer: 5'-cgtggcgggctaataacgtaNNKatgacgtcattgggtgaaatgc-3', capital letters are mutant bases,
[0030] Reverse primer: 5'-gcatttcacccaatgacgtcatMNNtacgttattagcccgccacg-3', capital letters are mutant bases;
[0031] The site-directed ...
Embodiment 2
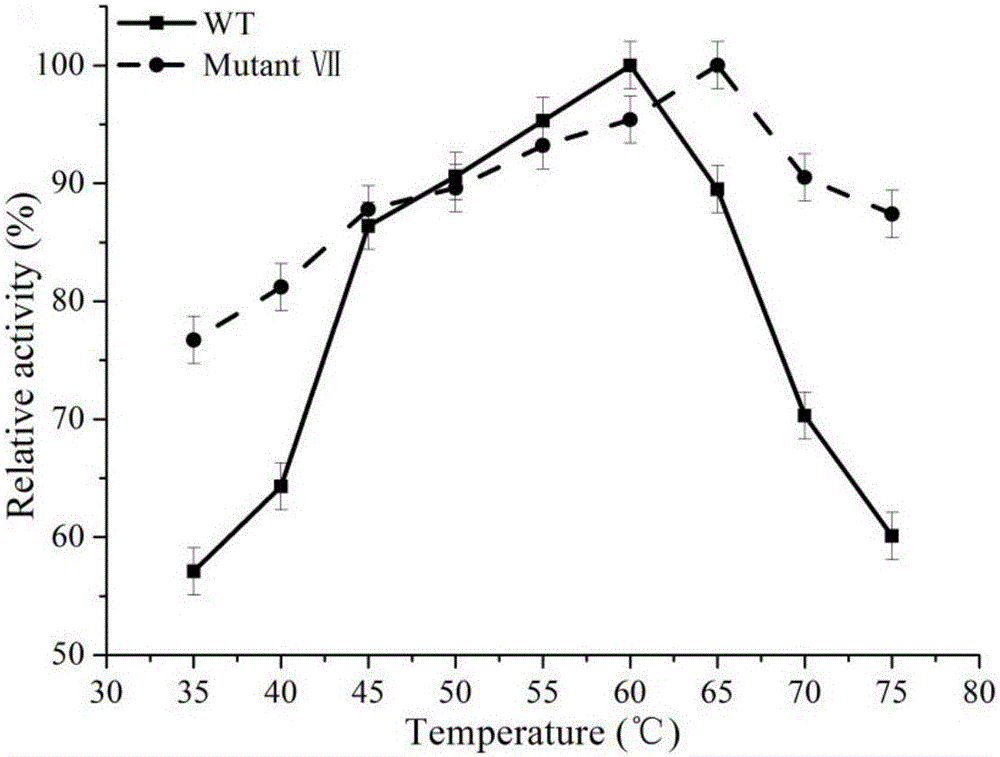
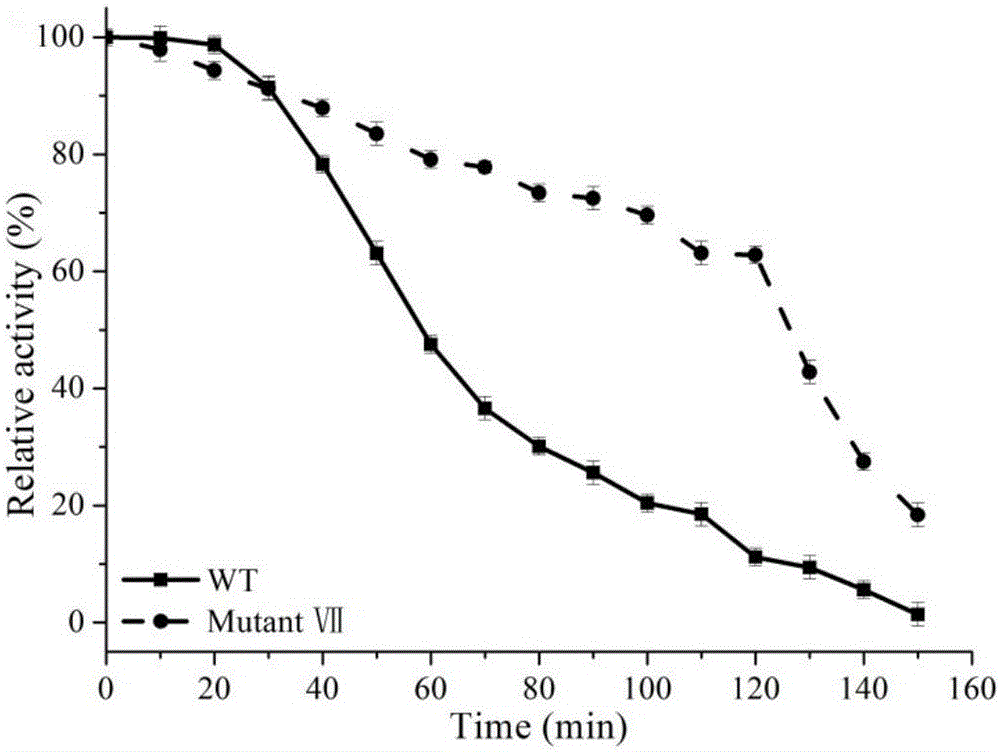
[0044] Example 2 Induction, expression and screening of highly thermostable β-glucanase
[0045] (1) Induction and expression of β-glucanase
[0046] Pick a single colony from the plate and insert it into a 96-well plate containing 250 μL LB medium (containing 50 μg / mL kanamycin sulfate). Each well corresponds to a specific transformant and a wild-type clone is inoculated in each 96-well plate as a negative control. The obtained 96-well plate was placed in a shaker at 37° C. and cultured at 200 rpm for 11-12 hours. Use a row pipette to draw 20 μL of the cultured bacterial solution and transfer it into a 96-well plate containing 250 μL of fresh LB medium, and store the remaining bacterial solution at 4°C for temporary storage. After culturing at 37°C and 200rpm for 4 hours, IPTG and α-lactose with final concentrations of 0.06mM and 8mM were added to each well respectively and the culture temperature was lowered to 24°C for 6 hours. The OD of the obtained bacterial solution w...
Embodiment 3
[0051] Embodiment 3 Enzyme activity and protein concentration analysis
[0052] (1) Enzyme activity assay method:
[0053] 3,5-Dinitrosalicylic acid (DNS) method combined with improved AZO assay method for the determination of β-glucanase activity:
[0054] Enzyme activity definition: 1 mL of enzyme solution under the conditions of 40°C and pH value of 6.5, the amount of hydrolyzing β-glucan per minute to produce glucose reducing substances equivalent to 1 μmol is 1 enzyme activity unit, expressed in U / mL.
[0055] Determination of the enzyme activity of the fermentation broth: after the fermentation broth is centrifuged, the supernatant is diluted to an appropriate multiple to measure its enzyme activity.
[0056] Drawing of glucose standard curve: draw 1% glucose standard solution 2.0, 3.0, 4.0, 5.0, 6.0mL respectively into 50mL volumetric flask, dilute to the mark with distilled water, and make each milliliter contain glucose 200, 400, 600, 800 , 1000, 1200μg dilute stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com