Polyacrylate-based polymer electrolyte for sodium battery and polymer sodium battery formed from polyacrylate-based polymer electrolyte
A polyacrylate and polymer technology, used in non-aqueous electrolyte batteries, electrolyte battery manufacturing, solid electrolytes, etc., can solve the problems of spontaneous combustion of batteries, hidden safety hazards of sodium batteries, and arbitrary shape restrictions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
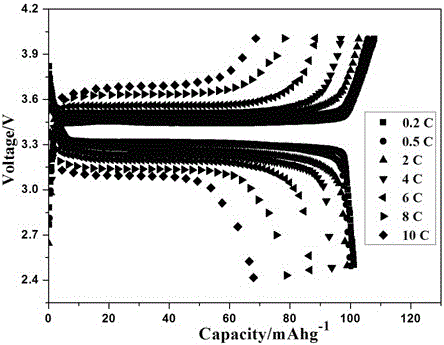
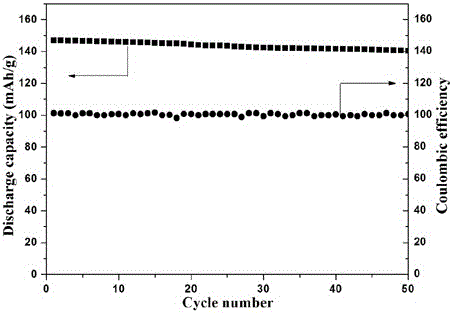
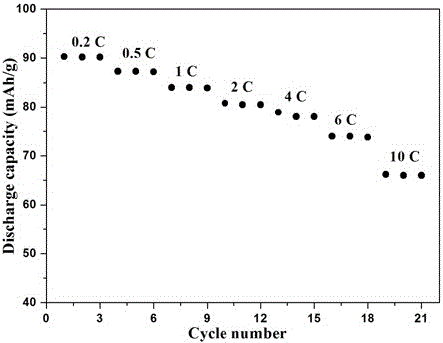
[0024] Dissolve 1.5g of polyethylene glycol diacrylate, 0.01g of azobisisobutyronitrile and 5g of sodium perchlorate in 75g of propylene carbonate, stir at room temperature until it is in a homogeneous solution state, and mix the above prepolymer solution, glass Fibrous separator, sodium vanadium phosphate positive electrode and sodium metal are assembled into a sodium battery, and after sealing, the o The polymer electrolyte sodium battery was prepared by in-situ polymerization at C for 5 hours.
[0025] After testing, the polymer electrolyte at 25 o The ionic conductivity at C is 8×10 -4 S / cm.
[0026] Test the electrochemical window of the prepared polymer electrolyte: sandwich the electrolyte with stainless steel sheets and sodium sheets, and place it in a 2032 battery case. The electrochemical window is measured by linear voltammetry scanning with an electrochemical workstation, the initial potential is 2.5V, the highest potential is 5.5V, and the scanning speed is 1mV...
Embodiment 2
[0028] Dissolve 2.5g of polyethylene glycol dimethacrylate, 0.02g of benzoyl peroxide and 8g of sodium hexafluorophosphate in a mixed liquid of 85g of ethylene carbonate and dimethyl carbonate (mass ratio 1:1), at room temperature Stir until it is in the state of a homogeneous solution, assemble the above-mentioned prepolymer solution, cellulose separator, molybdenum disulfide negative electrode and sodium metal into a sodium battery, seal it well, and set it at 70 o C in situ polymerization for 4 hours to prepare a polymer electrolyte sodium battery.
[0029] After testing, the polymer electrolyte at 25 o The ionic conductivity at C is 4×10 -4 S / cm.
[0030] Test the electrochemical window of the prepared polymer electrolyte: sandwich the electrolyte with stainless steel sheets and sodium sheets, and place it in a 2032 battery case. The electrochemical window is measured by linear voltammetry scanning with an electrochemical workstation, the initial potential is 2.5V, the ...
Embodiment 3
[0032] Dissolve 1.5g of triethylene glycol diacrylate, 0.03g of azobisisoheptanonitrile and 6g of sodium hexafluorophosphate in 87g of propylene carbonate, stir at room temperature until it is in a homogeneous solution state, and mix the above prepolymer solution, nylon Porous separator, sodium vanadium phosphate positive electrode and molybdenum disulfide negative electrode are assembled into a sodium battery, and after sealing, the o C in situ polymerization for 2 hours to prepare a polymer electrolyte sodium battery.
[0033] After testing, the polymer electrolyte at 25 o The ionic conductivity at C is 6.2×10 -4 S / cm.
[0034] Test the electrochemical window of the prepared polymer electrolyte: sandwich the electrolyte with stainless steel sheets and sodium sheets, and place it in a 2032 battery case. The electrochemical window is measured by linear voltammetry scanning with an electrochemical workstation, the initial potential is 2.5V, the highest potential is 5.5V, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com