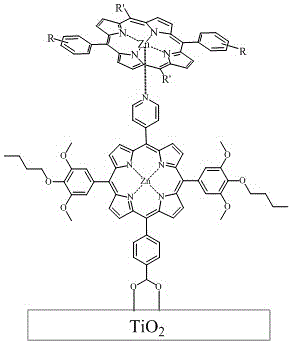
Ferrocene zinc porphyrin organic dye and synthesis as well as application thereof to preparation of dye-sensitized solar cell
A technology of organic dyes and ferrocene, applied in the fields of organic dyes, organic chemistry, azo dyes, etc., can solve the problems of high raw material cost, limited efficiency improvement potential and complicated production process of silicon-based batteries, and improve the electron transmission efficiency. , The effect of improving photoelectric conversion efficiency and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
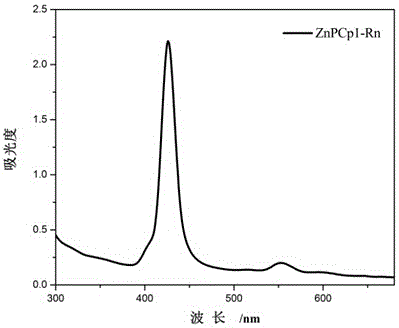
[0058] Embodiment 1, the preparation and application of ZnPCp1-Rn
[0059] 1. Preparation of ZnPCp1-Rn
[0060] (1) Synthesis of 5,15-bis(4-nitrobenzene)-10,20-bis(3,5-dimethoxy-4-butoxybenzene)porphyrin: 1mmol 5-p-nitrobenzene Base dipyrromethane and 1mmol 3,5-dimethoxy-4-butoxybenzaldehyde were added to 100mL CH 2 Cl 2 , reacted at room temperature for 15 min under the protection of argon, then added 1.5 mmol TFA, and stirred at room temperature for 30 min under the protection of argon. Then add 0.57gDDQ, continue to react for 1h. Dichloromethane washes away the impurity layer, collects all liquids and evaporates to dryness, adds DDQ toluene solution (100mL toluene and 0.57gDDQ), continues reflux reaction for 1h, evaporates to dryness, separates by column chromatography, and uses petroleum ether and ethyl acetate as Eluent, collect the main color bands, dry, and obtain a purple powder product;
[0061] (2) Synthesis of 5,15-bis(4-aminobenzene)-10,20-bis(3,5-dimethoxy-4-...
Embodiment 2
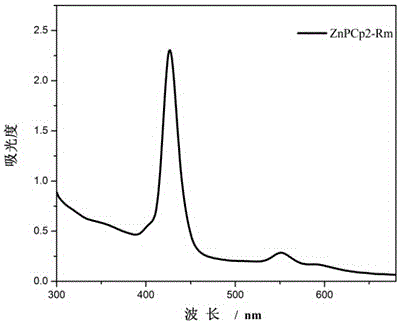
[0071] Embodiment two, the synthesis and application of ZnPCp2-Rm
[0072] 1. Synthesis of ZnPCp2-Rm
[0073] Steps (1), (2), and (3) are the same as in Embodiment 1.
[0074] (4) Synthesis of 5,15-bis[4-(ferrocenyliminophenyl)]10,20-bis(3,5-dimethoxy-4-butoxybenzene) zinc porphyrin: Dissolve 1 mmol of 5,15-bis(4-aminobenzene)-10,20-bis(3,5-dimethoxy-4-butoxybenzene) zinc porphyrin and 9 mmol of ferroceneformaldehyde in 20 mL of N,N- In dimethylformamide, under the protection of argon, react at 80°C for 24h; after the reaction, cool to room temperature, add water and filter to obtain the crude product, wash the filter cake with water and methanol successively, and dry to obtain the target product ZnPCp2-Rm. The yield was 46%.
[0075] (5) Use a Varian nuclear magnetic resonance instrument (400M) to detect the product, and the nuclear magnetic data of the dye ZnPCp2-Rm: 1 HNMR (400MHz, CDCl 3 ):δ8.98(s,4H,βH),8.82(s,4H,βH),8.69(s,2H,N=CH-),8.58(d,4H,ArH),8.31(d,4H,ArH ),7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com