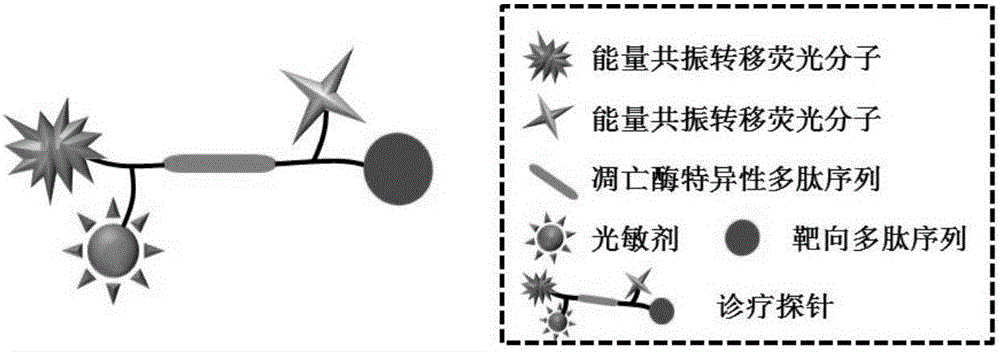
Tumor-targeted diagnosis and treatment integrated fluorescent probe
A tumor-targeting and probe technology, applied in the field of fluorescent probes for tumor-targeted diagnosis and treatment, can solve problems such as inability to feedback and monitor, and achieve the effects of improving solubility, simple preparation method, and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] [Example 1] Synthesis of fluorescent probes for tumor targeting diagnosis and treatment
[0049] Protoporphyrin-Lysine (Fluorescein)-Serine-Aspartic Acid-Glutamic Acid-Valine-Aspartic Acid-Serine-Lys (Dimethylaminoazobenzene)-Arginine- Synthesis of Glycine-Aspartic Acid, PpIX-K(FAM)SDEVDSK(Dabcyl)RGD) at room temperature:
[0050] (1) Add 0.5g of ammonia resin (0.525mmol / g) to the reactor containing 10mL of re-steamed N,N-dimethylformamide, and wait until the ammonia resin is in N,N-dimethylformamide After swelling for 2h at room temperature, N,N-dimethylformamide was removed.
[0051] (2) Add 10 mL of 20% (V / V) piperidine / N,N-dimethylformamide (that is, the volume ratio of piperidine to N,N-dimethylformamide is 2:8) into the reactor. After reacting at room temperature for 15 minutes, remove the solvent; repeat the reaction by adding piperidine / N,N-dimethylformamide solution to cut off the FMOC protecting group. After the reaction, remove the solvent and use N,N-dimethylform...
Embodiment 2
[0063] [Example 2] Detection of the response of a fluorescent probe for tumor targeting diagnosis and treatment to apoptotic enzyme-3
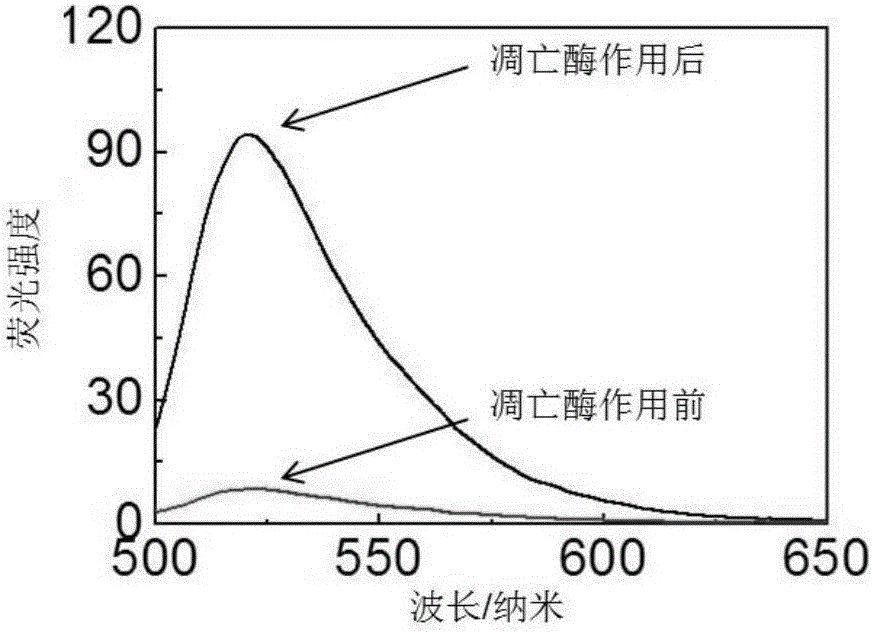
[0064] Dissolve the probe in the HEPES buffer solution and configure it as a working solution of 1 μmol / L. The apoptotic enzyme-3 (1U) was added to the buffer solution containing the probe, and the working solution was diluted with the buffer solution to a final concentration of 0.5 micromol / L. Fluorescence spectrometer (LS55 fluorescence spectrophotometer, Perkin-Elmer) was used to detect the fluorescence intensity of luciferin in the solution immediately after the apoptotic enzyme-3 was added and 11 hours after the apoptotic enzyme-3 was added. The excitation wavelength of fluorescein is: 465 nm.
[0065] The result is figure 2 As shown, the luciferin intensity of the probe in the solution was weak when the apoptotic enzyme-3 was just added, and after 11 hours of interaction with the apoptotic enzyme-3, the luciferin intensity of the probe was ...
Embodiment 3
[0066] [Example 3] Specific detection of the response of apoptotic enzyme-3 by fluorescent probes for tumor targeting diagnosis and treatment
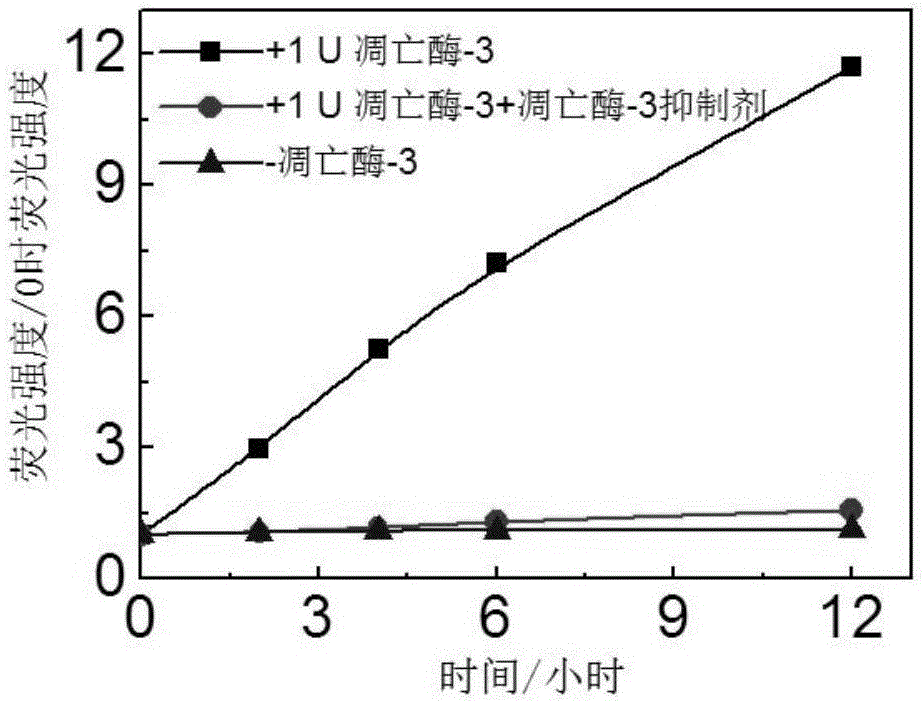
[0067] The apoptotic enzyme-3 (1U) was incubated with a commercial apoptotic enzyme-3 specific inhibitor (Ac-DEVD-CHO, 50 μmol / L) at 37 degrees Celsius for 2 hours. The probe solution was prepared into a working solution of 1 micromol / L in the HEPES buffer solution. Add apoptotic enzyme-3 (1U) and apoptotic enzyme-3 (1U) incubated with the apoptotic enzyme inhibitor to the buffer solution containing the probe, and dilute the probe concentration with the buffer solution to a final concentration of 0.5 μmol / Rise. Use a fluorescence spectrometer to record how the fluorescence of the probe solution without apoptotic enzyme-3, apoptotic enzyme-3, and apoptotic enzyme-3 and inhibitors changes over time. The excitation wavelength of fluorescein: 465 nm; the emission wavelength of collected fluorescein: 520 nm.
[0068] The result is image 3 A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com