Lipoic-acid-modified nanometer polypeptide carrier and preparation method and application thereof
A kind of lipoic acid and nano technology, which is applied in the field of medicine and achieves the effects of low composition, low cytotoxicity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Synthesis of lipoic acid-modified 9-peptide
[0063] The 9-peptide amino acid sequence modified by lipoic acid (LA): HisHisHisArgArgArgArgArgArg (His, histidine; Arg, arginine) (SEQ ID NO: 1), LA-HR, was synthesized by Shanghai Jier Biochemical Co., Ltd. by peptide solid-phase synthesis and named LA-H 3 R 6 , the synthetic LA-HR was purified by preparative high-performance liquid chromatography, and its purity reached more than 95%. LA- is lipoic acid, R is arginine, H is histidine, and the amino acids are connected by peptide bonds to form a 9-peptide.
Embodiment 2
[0064] Example 2: Preparation of lipoic acid-modified polypeptide nanocarrier LAHRss
[0065] Dissolve 50 mg of lipoic acid-modified polypeptide LAHR and different amounts of cysteine hydrochloride in 10 ml of methanol, add sodium hydroxide solution to adjust the pH to 7.0, stir and react for 12 hours, and the reaction temperature is 10-30°C. The amount of cysteine is respectively: 2.5%, 5%, 10%, 20%.
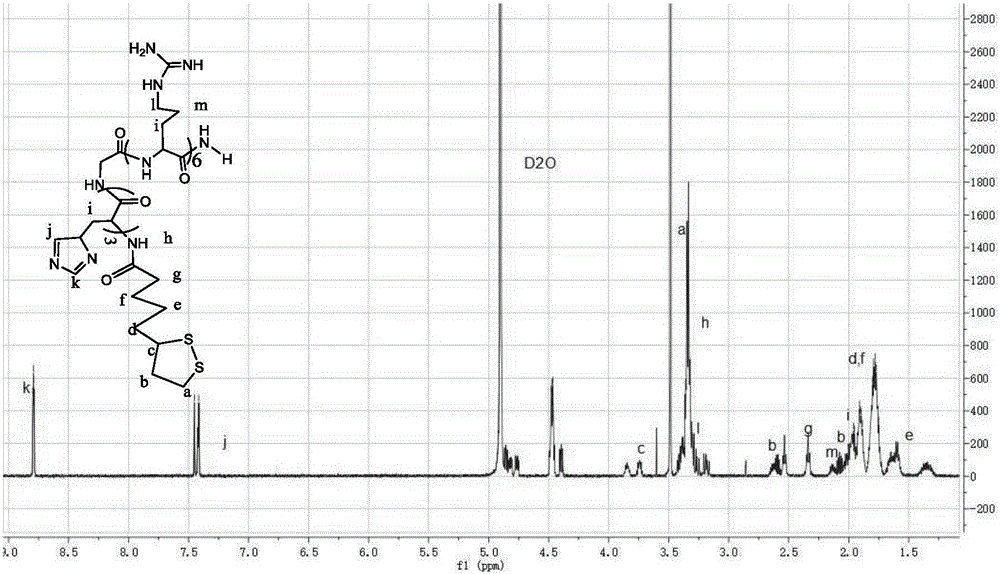
[0066] As shown in Table 1, LAHRss with different molecular weights were prepared according to the ratio of LAHR to cysteine. The obtained solution was dialyzed for 12 hours through a dialysis bag with a molecular weight cut-off of 1000. The dialysate was distilled water, and the dialysate was replaced every 4 hours. The obtained dialysis product was lyophilized and stored at -20°C, and the lyophilized carrier could be stored for a long time under reconstitution conditions. The synthesized carrier was subjected to H NMR spectroscopy 1 H-NMR (600M) detection, molecular we...
Embodiment 3
[0071] Example 3: Preparation of LAHRss in pDNA Nanomicelles
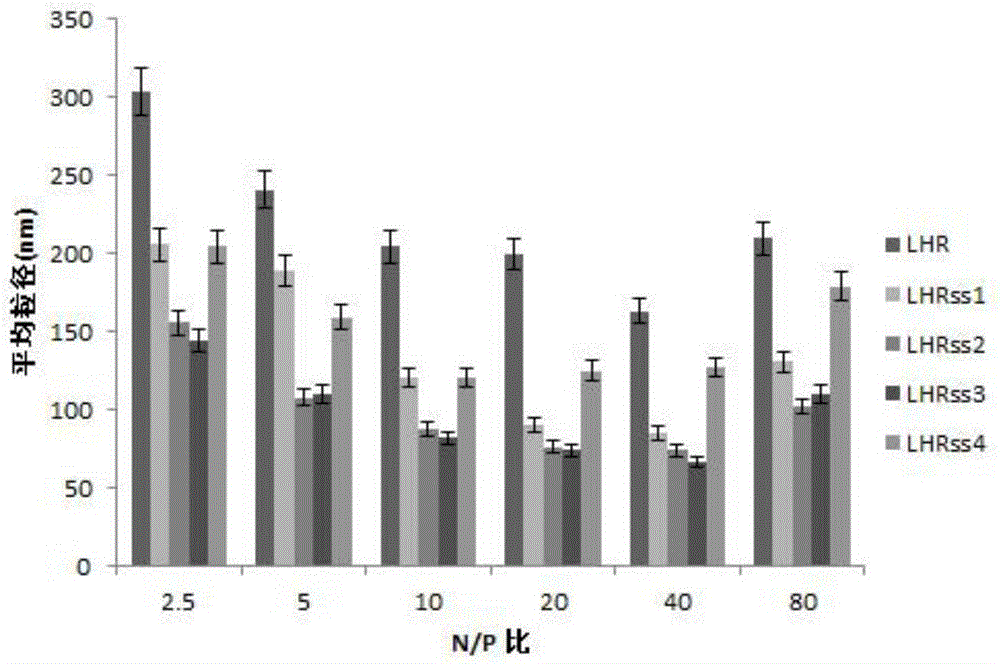
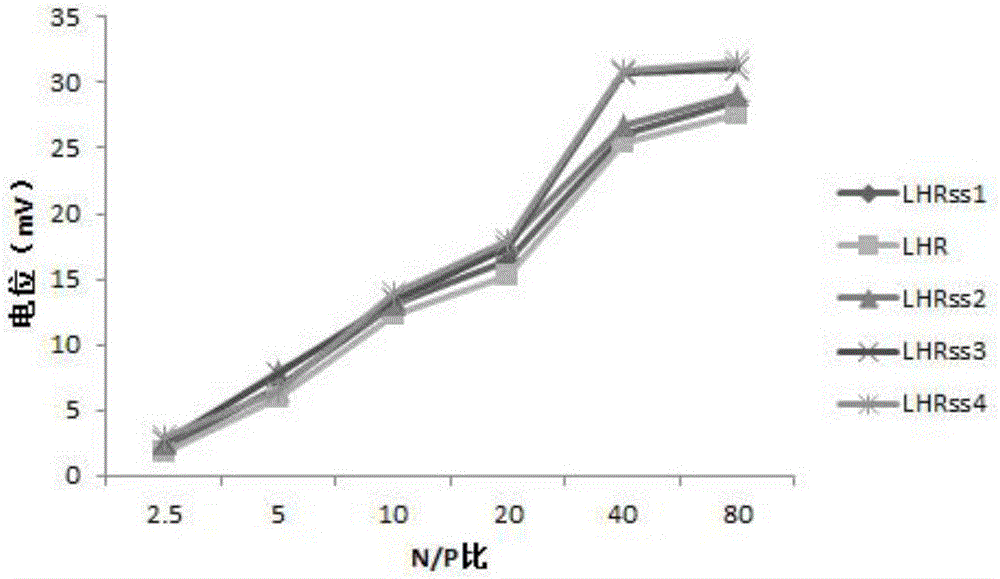
[0072] The carrier (LAHRss) and luciferase expression plasmid pEGFP (Shanghai Innovation Biotechnology Co., Ltd.) were dissolved in water to prepare aqueous solutions, and the nitrogen-phosphorus ratio (N / P) was 2.5, 5, 10, 20, 40, 80, respectively. The nanocomposites were prepared and vortexed for 10 seconds, then left to stand for 30 minutes to obtain nanomicelles. The average particle size of nano micelles is related to N / P. When N / P=40, the best particle size is obtained, and the particle size is between 80-300. For details, see figure 2 . The Zeta potential of nanomicelles increases with the increase of N / P, and is stable at 0-30mV when N / P is greater than 2.5, see image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com