Pharmaceutical composition for treating heart failure with preserved ejection fraction and application of pharmaceutical composition
A heart failure and ejection fraction technology, applied in the field of medicine, can solve the problems of reducing myocardial infarction size and increasing the release of vascular endothelial relaxation factors, and achieve the effect of increasing patient compliance, reducing treatment costs, and reducing drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Fifteen healthy Wistar male rats, weighing 200-220 g. After one week of adaptive feeding of the rats, 3 rats were randomly selected as the sham operation group (the surgical silk thread was passed through the abdominal aorta after laparotomy in the sham operation group, but the abdominal aorta was not ligated), and the remaining 12 rats were treated as follows: Aortic coarctation surgery to establish an animal model of heart failure with preserved ejection fraction: After skin preparation, the rats were anesthetized intraperitoneally with 3% sodium pentobarbital at 2.3ml / kg, made a midline incision under the xiphoid process, and opened the abdominal cavity in layers. Bluntly dissect the abdominal aorta below the branch of the renal artery, place the needle of a No. 9 syringe parallel to the abdominal artery, ligate the abdominal aorta and the needle with No. 4 surgical silk thread, and then withdraw the needle slowly to make the rat abdominal aorta The diameter was redu...
Embodiment 2
[0029] The method is the same as in Example 1, except that the Alda-1 group is given 6 mg / kg of Alda-1 by stomach every day; the combined drug group is given 3 mg / kg of Alda-1 and 15 mg / kg of isosorbide mononitrate by stomach every day .
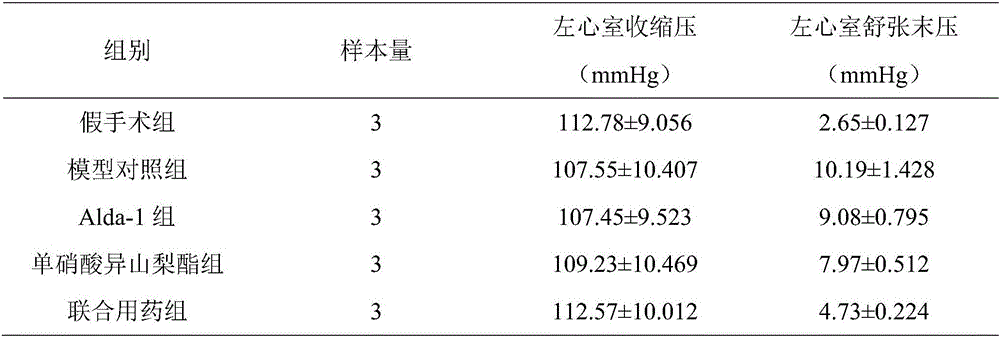
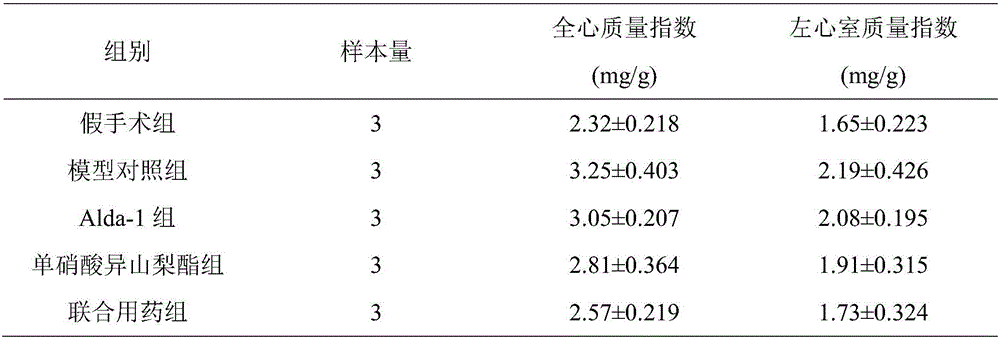
[0030]The left ventricular systolic pressure and left ventricular end-diastolic pressure were detected by hemodynamics. The statistical results are shown in Table 1. Myocardial hypertrophy index was measured for whole heart mass index and left ventricular mass index, and the statistical results are shown in Table 2.
[0031] It should be noted that the rats in the model control group did not take any treatment measures, and the symptoms of heart failure gradually aggravated, and all died after 5 days. The symptoms of heart failure in rats in the combined drug group were significantly improved, and the concomitant symptoms of rats in the special combined drug group were improved most significantly. In addition, after taking the drug for 10 ...
Embodiment 3
[0040] The method is the same as in Example 1, except that the Alda-1 group is administered intragastrically with 90 mg / kg of Alda-1 every day; the combined drug group is administered with 45 mg / kg of Alda-1 and 15 mg / kg of isosorbide mononitrate .
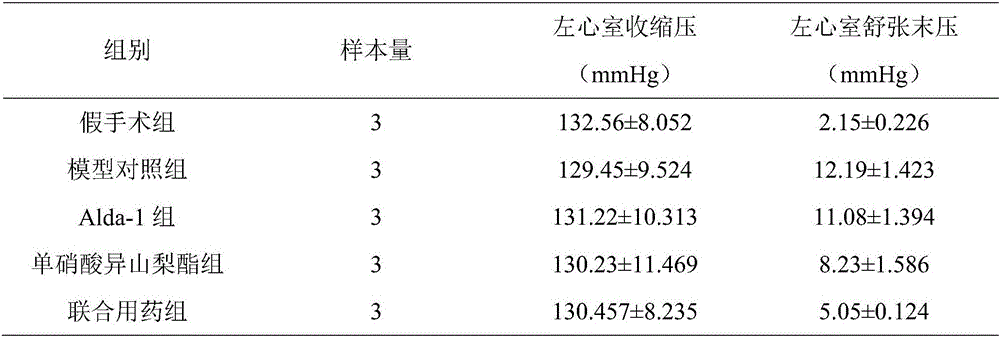
[0041] The left ventricular systolic pressure and left ventricular end-diastolic pressure were detected by hemodynamics. The statistical results are shown in Table 1. Myocardial hypertrophy index was measured for whole heart mass index and left ventricular mass index, and the statistical results are shown in Table 2.
[0042] It should be noted that the rats in the model control group did not take any treatment measures, the symptoms of heart failure gradually aggravated, and all died after 10 days. The Alda-1 group had no obvious effect on the improvement of the rats' heart failure symptoms. The symptoms of heart failure in the combined drug group were significantly improved, and the concomitant symptoms of heart failure in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com