DNA molecule for coding porcine alpha interferon and expression and purification method of recombinant protein of porcine alpha interferon
An interferon and coding technology, applied in the field of protein purification in the large-scale production of recombinant interferon, can solve the problems of complex steps and low antiviral activity of recombinant porcine alpha-interferon, and achieve a short purification period and disulfide bond. Correct pairing, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
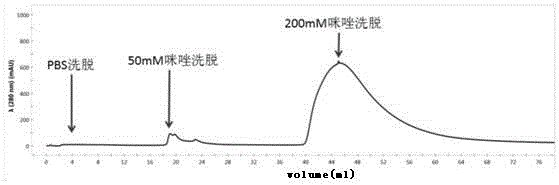
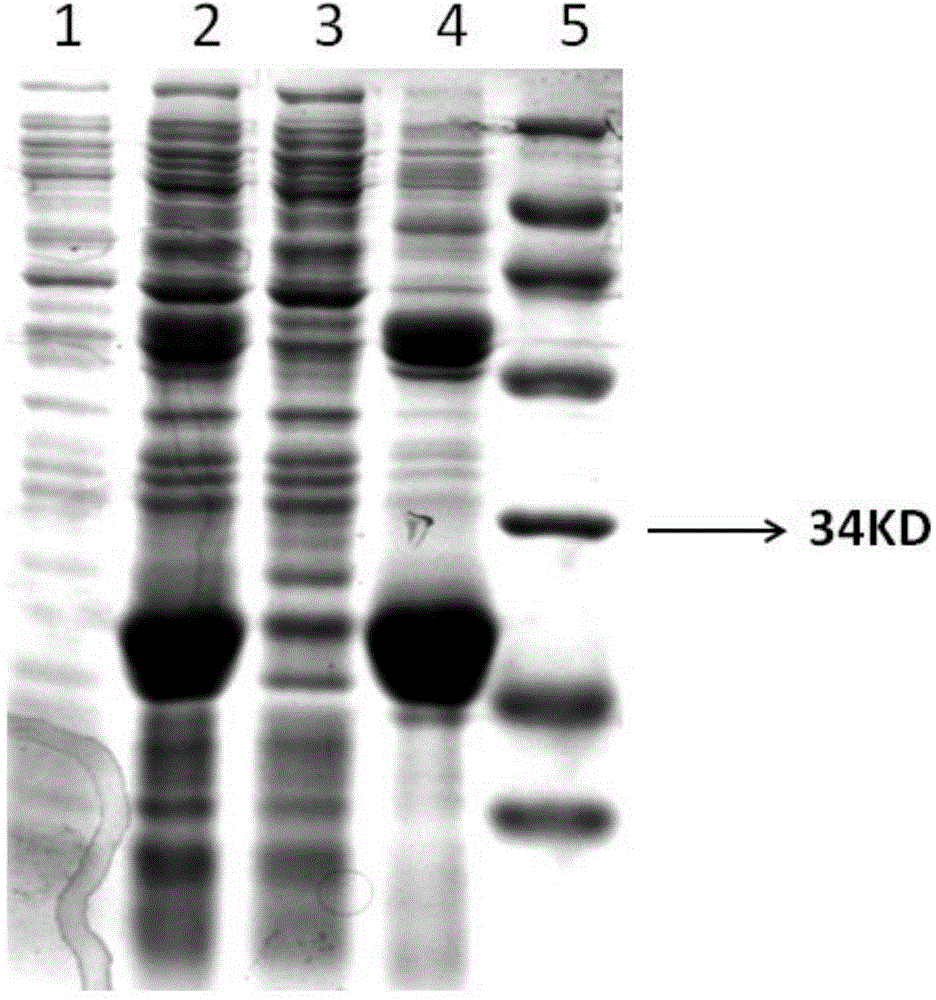
[0046] Construction, expression and purification of embodiment 1 recombinant porcine interferon alpha
[0047] (1) Optimization of porcine interferon-α gene and construction of seed bacteria: The porcine interferon-α gene was optimized and transformed according to the codon preference of Escherichia coli, and the transformed gene was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. and constructed into pET21a On the vector, the recombinant expression plasmid pET-21a-rPoIFNα was obtained. Its nucleotide sequence is shown in SEQIDNo.1, including 519 bases, the 1st-516th base encodes 172 amino acids, and its theoretical molecular weight is 19.8kDa. The expression plasmid pET-21a-rPoIFNα was transformed into Escherichia coli BL21 competent cells to obtain the expression seed strain BL21 / pET-21a-rPoIFNα.
[0048] (2) Seed fungus recovery: Inoculate the seed fungus frozen at -20°C on the ampicillin-resistant LB solid medium with an inoculation loop, and cultivate it in a con...
Embodiment 2
[0059] Antiviral biological activity assay of embodiment 2 recombinant porcine alpha interferon protein
[0060] Biological activity was determined using a cytopathic (CPE) inhibition-based microinhibition assay. Using the Marc-145 cell-porcine reproductive and respiratory syndrome virus (PRRSV) system, the highest dilution of interferon that inhibited 50% cytopathic changes was defined as 1 interferon activity unit.
[0061] (1) Inoculate Marc-145 cells into a 96-well plate (100 μl / well), culture for 6-8 hours until all adhere to the wall, and make it reach 10 4 pcs / hole or so;
[0062] (2) Discard the culture medium, dilute the prepared recombinant porcine interferon alpha protein sample for 8 gradients, the dilution solution is DMEM medium containing 10% FBS, set 8 replicates for each gradient, and add 100 μl virus to each well Diluent.
[0063] (3) After culturing at 37°C for 18 hours, add PRRSV virus solution (300TCID50 / well) diluted with serum-free DMEM medium, and se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com