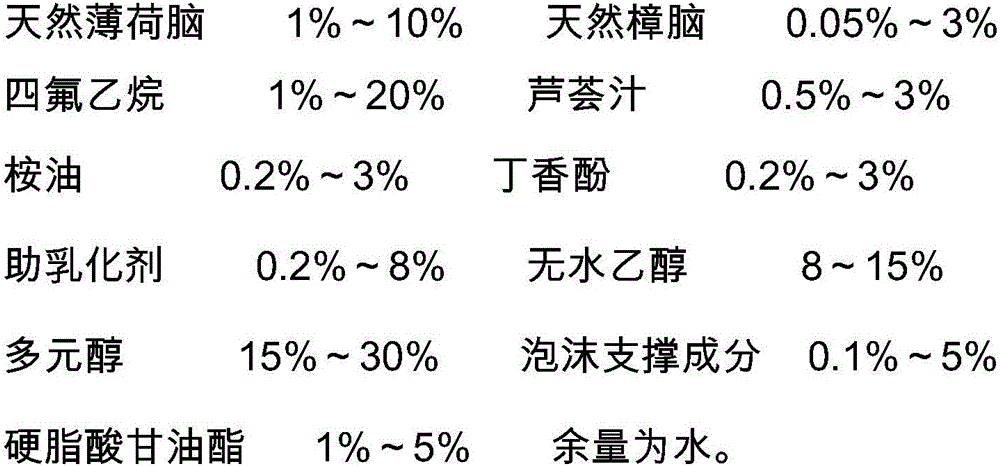
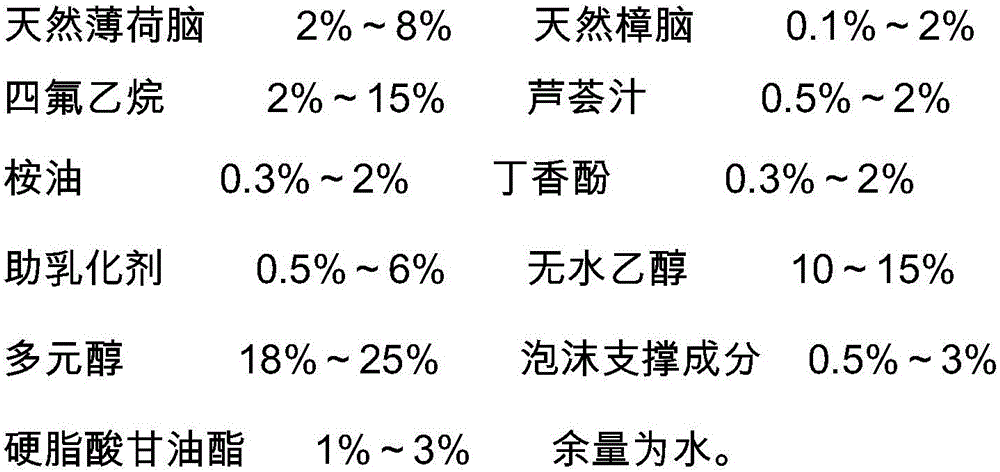
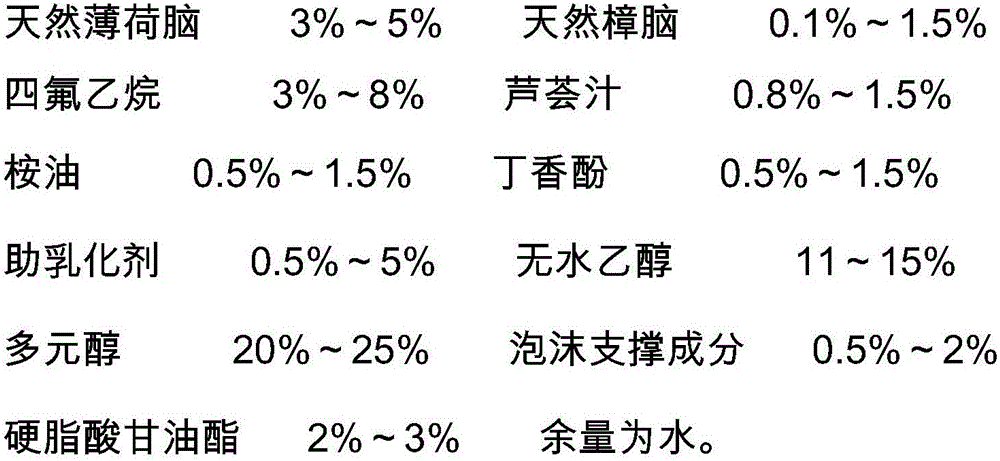
Foaming agent with itching-relieving and inflammation-diminishing effects and preparation method of foaming agent
A foaming agent and technology of efficacy, applied in the field of foaming agent and its preparation, can solve the problems of incomplete absorption, skin discomfort, unsustainable effect, etc., and achieve the effects of enhancing the use effect, enhancing the cooling effect and the soft product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the selection of co-emulsifier
[0062] 1.1 Experimental method: Sodium lauryl sulfate, Tween-80, polyoxyl (40) stearate, triethanolamine, polyoxyethylene cetyl ether, alkyl glucoside, sorbitan lauric acid Equal amounts of the esters were weighed, and the Ross-Miles method was used, and the foam height change within 30 minutes after foaming was used as the evaluation index of foam stability. At the same time, since the formula of the foam agent matrix is similar, the matrix formula of this patent is used as the matrix, and different surfactants are added to it, mixed evenly, poured into the aluminum tube, added with the same amount of propellant, and packaged. A sample size of 50 people was selected to conduct sensory tests on the foam softness and skin irritation of foams prepared with different surfactants.
[0063] 1.2 Ross-Miles determination of foam performance: At 40°C±0.5°C, 200mL sample solution flows down from a pore with a height of 900mm and ...
Embodiment 2
[0076] Embodiment 2: antipruritic effect experiment
[0077]1. Experimental method: get 50 guinea pigs, be divided into 5 groups at random: blank control group, positive control group (for Mentholatum Peppermint Ointment, dosage is 5ml / kg) and foam agent high, middle and low dosage groups of the present invention (dose respectively 10, 5, 2.5ml / kg), 10 rats in each group. Guinea pigs were depilated with 8% barium sulfide on their backs, and 24 hours later, the corresponding test drugs were administered percutaneously, and the blank control group was given corresponding blank matrix, once a day, for 5 consecutive days. 60 minutes after the last administration, scratch the shaved area with coarse sandpaper to make it red, with an area of about 1cm×1cm, and add 0.01% histamine phosphate solution 0.05ml dropwise on the wound surface, and then Every 3min with 0.02%, 0.03%, 0.04%.. the concentration is increasing, each time 0.05ml per drop, until the guinea pig turns back to lick...
Embodiment 3
[0085] Embodiment 3: anti-inflammatory effect experiment
[0086] 1.1 Experimental method: Take 50 male mice and randomly divide them into 5 groups, namely blank control group (with the same dose of blank matrix), high, medium and low dose groups (with doses of 10, 5, 2.5ml / kg respectively ), positive control group (aspirin 100mg / kg). Each group was smeared on the abdomen of the mice respectively for 5 consecutive days, and the weight of the mice was recorded every day. One hour after the last administration, the two sides of the right auricles of the mice in each group were evenly smeared with xylene (0.05ml each). After 4 hours, the mice were sacrificed by dislocation of the cervical vertebrae. The ear pieces were removed from the same part of both ears, weighed with an electronic analytical balance, and the swelling value was obtained by subtracting the weight of the left ear piece from the weight of the right ear piece.
[0087] 2. Experimental results
[0088] 2.1 Inhi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com