Supramolecular hydrogel factor, supramolecular hydrogel and preparation methods of supramolecular hydrogel factor and supramolecular hydrogel
A supramolecular hydrogel and factor technology, applied in gel preparation, chemical instruments and methods, colloid chemistry, etc., can solve the problem of low biological activity, achieve high reaction yield, simple operation, and good biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] One embodiment of the preparation method of supramolecular hydrogel factor and supramolecular hydrogel of the present invention comprises the following steps:
[0043] (1) Preparation of supramolecular hydrogel factors
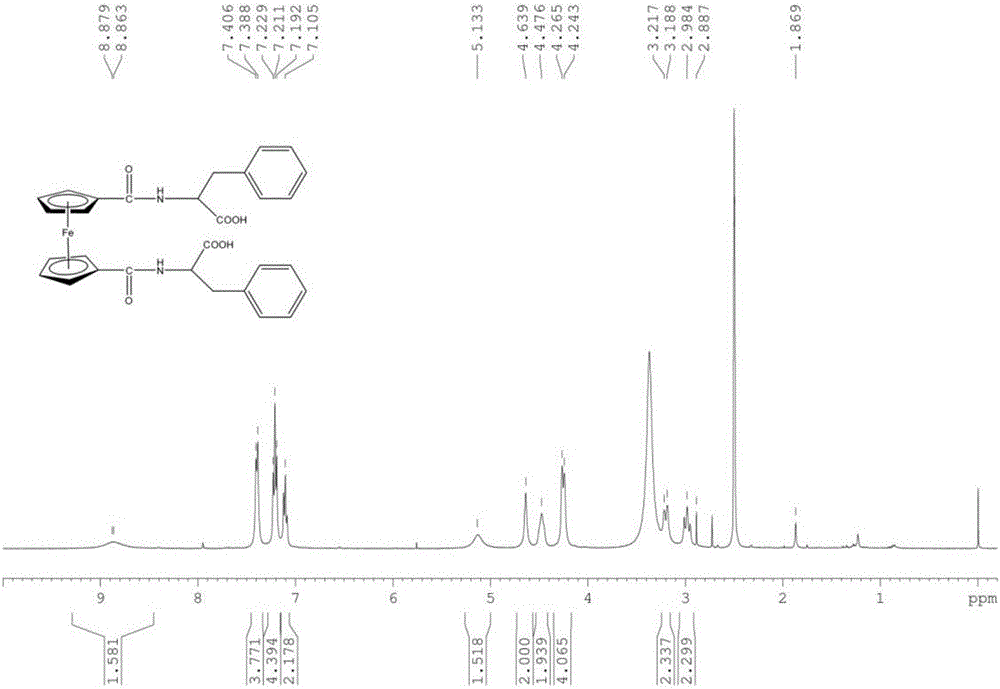
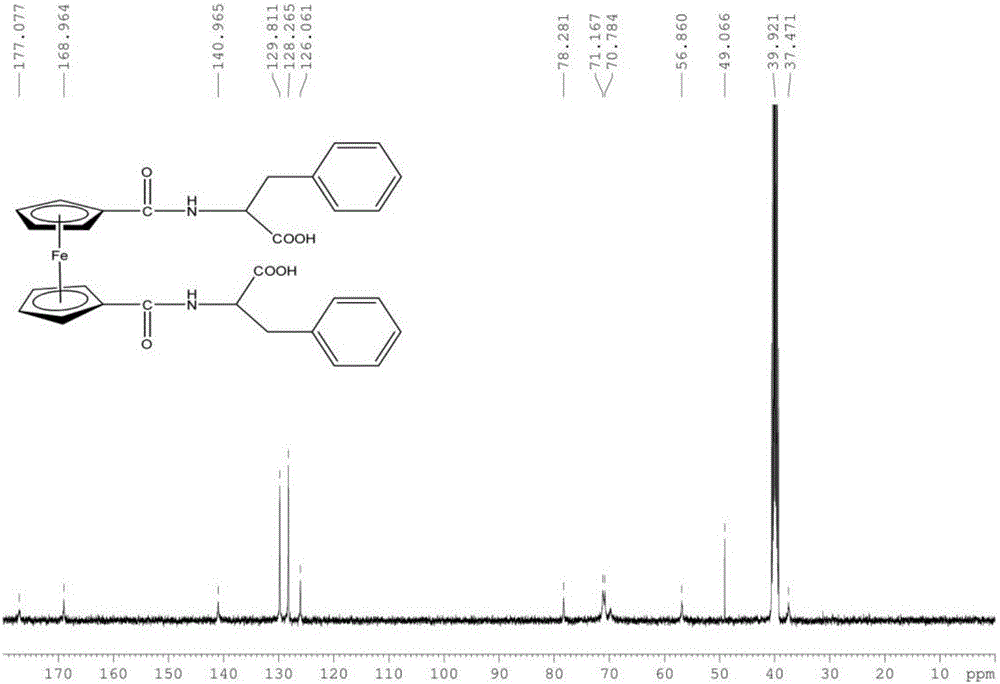
[0044] Weigh 1.84g (6.71mmol) of ferrocenedicarboxylic acid, dissolve it in 250mL of anhydrous dichloromethane, slowly add 1.20g (8.88mmol) of 1-hydroxybenzotriazole (HOBt), 3.36g (8.86 mmol) benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and 1.80g (9.99mmol) phenylalanine methyl ester hydrochloride (H-PHe- OMe·HCl). Slowly add about 6 mL of triethylamine dropwise to adjust the pH of the solution to 8-9. The reaction was stirred at room temperature for 12h. Reaction was followed by thin layer chromatography (TLC) spot plate. After the reaction is completed, successively use saturated Na 2 CO 3 The aqueous solution, hydrochloric acid with a mass fraction of 5%, and distilled water were each extracted once, and the extracted d...
Embodiment 2
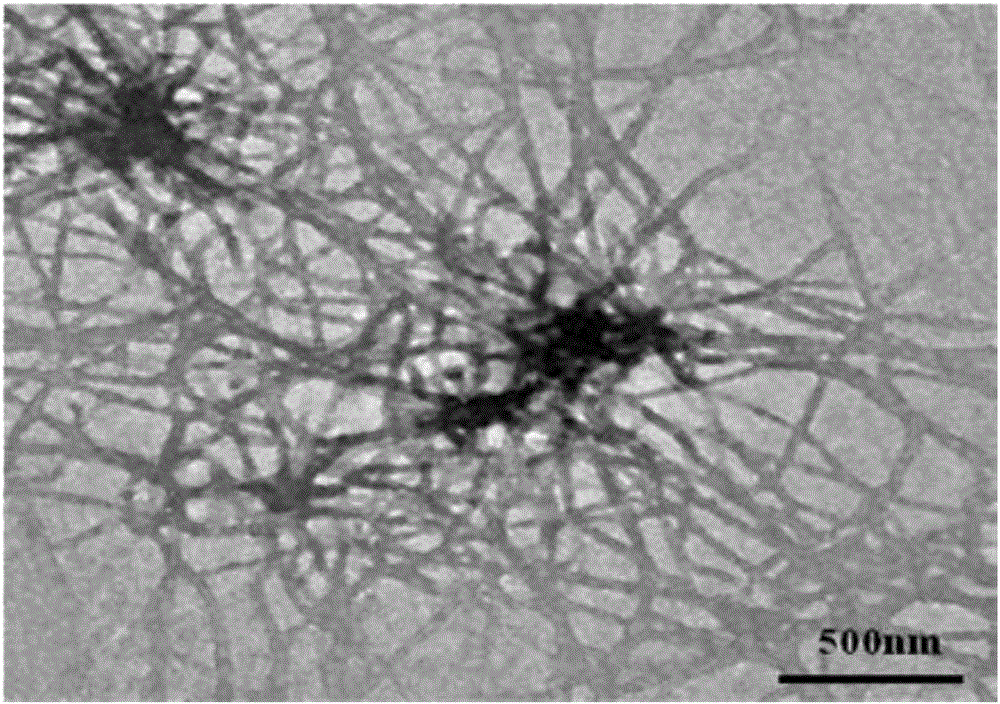
[0049] The glassy carbon electrode was polished with polishing powder (alumina powder), then ultrasonically washed twice with absolute ethanol and secondary water respectively, then rinsed with secondary water, and dried with nitrogen. A glassy carbon electrode was used as the working electrode, Ag / AgCl was used as the reference electrode, a platinum wire electrode was used as the counter electrode, and the electrolyte solution was PBS buffer solution (10 mmol / L, pH 7.4). At room temperature, with a scan rate of 0.05V / s, an initial potential of 0.3V, and a first reentry potential of 1.0V, the electrochemical data of the bare electrode was measured by cyclic voltammetry. The test results are shown in Figure 6 Curve 1. Rinse the above-mentioned glassy carbon electrode with secondary water, dry it with nitrogen, drop 3 μL of the gel prepared in Example 1 above on the glassy carbon electrode, dry it at room temperature, and use the above-mentioned method for measuring the bare el...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com