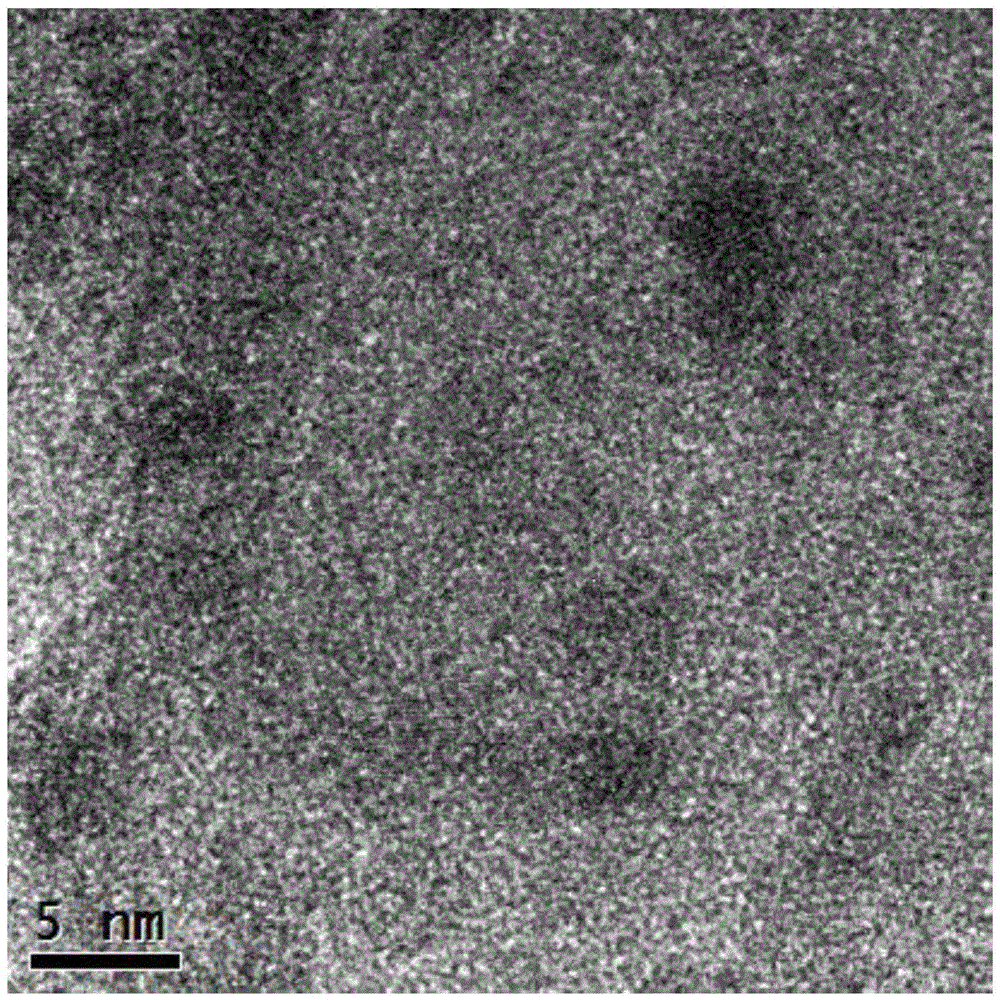
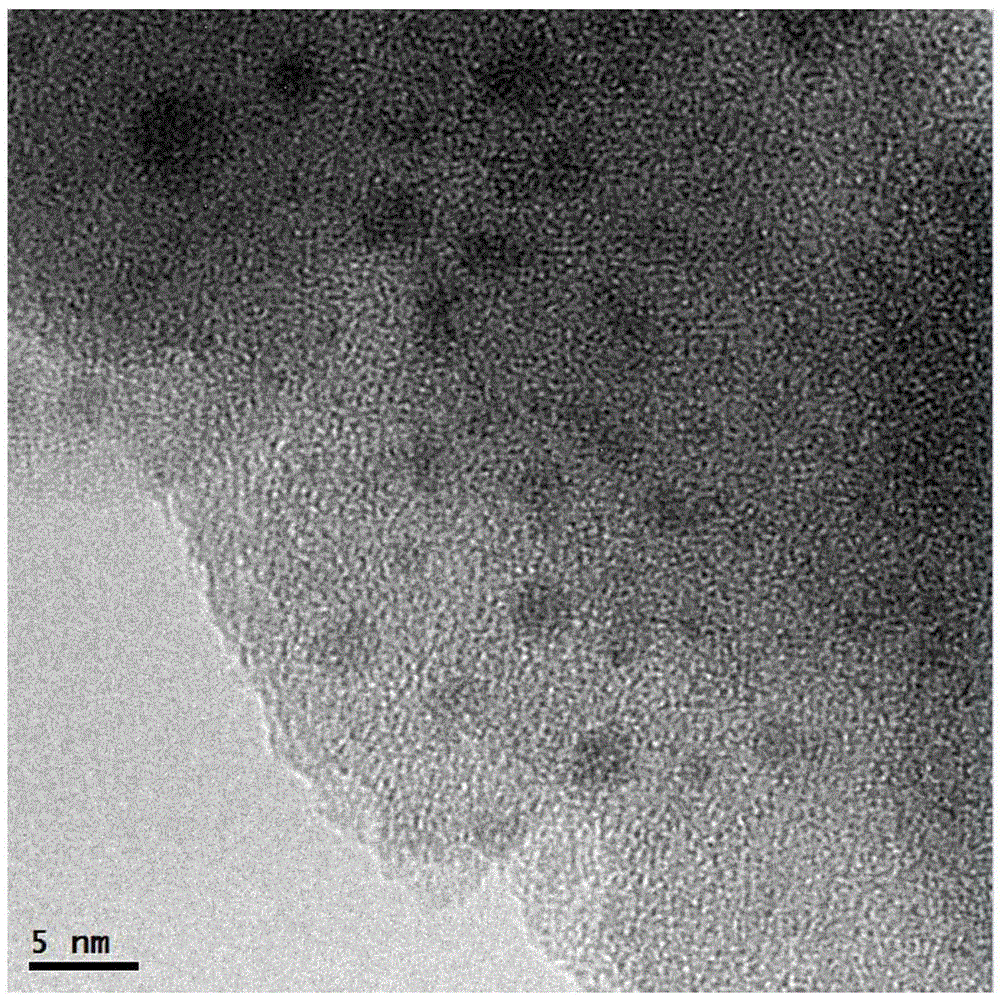
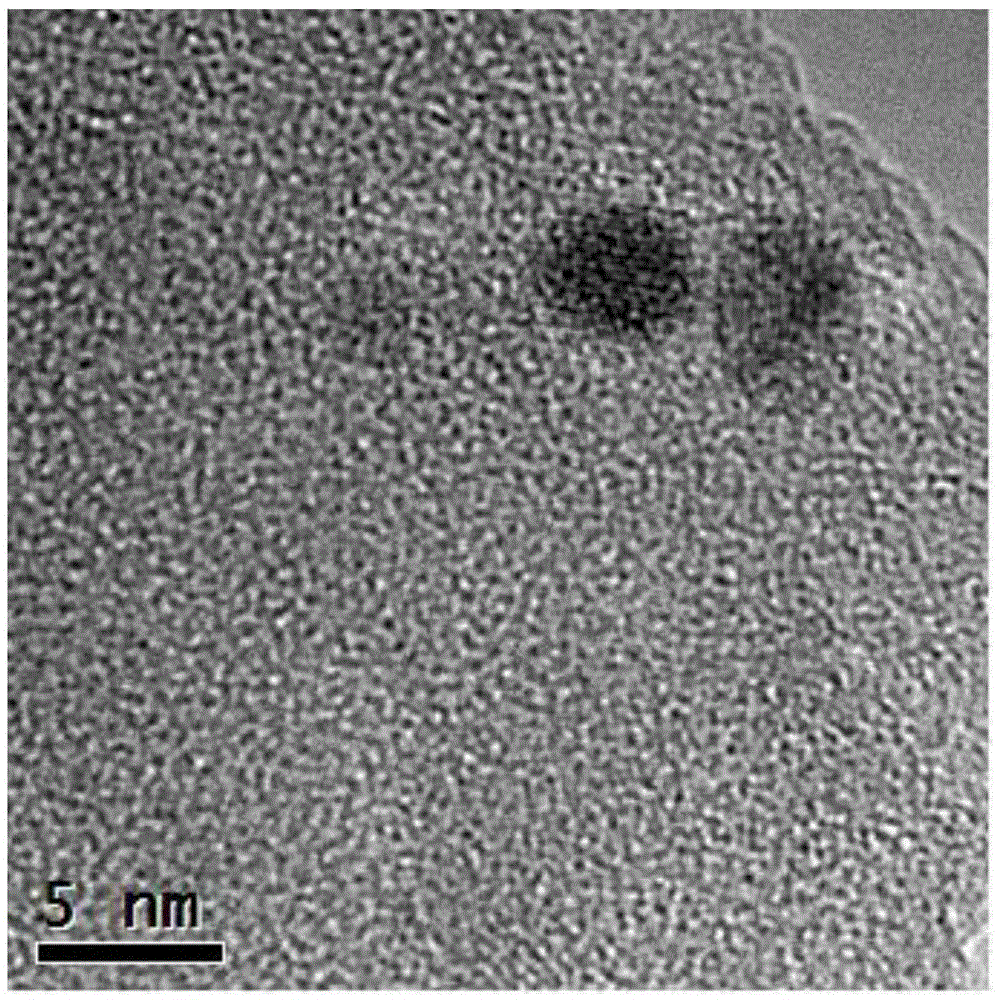
Catalyst used for synthesis of dimethyl oxalate and preparation method thereof
A technology for synthesizing dimethyl oxalate and dimethyl oxalate, applied in the directions of carbon monoxide or formate reaction preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of increasing the dechlorination process, the complicated catalyst preparation process, problems such as poor catalyst activity, to achieve the effect of reducing the dechlorination process, shortening the preparation cycle, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] According to the loading capacity of 0.3wt% Pd, measure 15mL of 0.04mol / L palladium acetate dichloromethane solution, add 10gα-Al 2 o 3 (specific surface area 3m 2 / g, particle size 2.5mm) soaked in this active ingredient solution for 12 hours, then transferred to a 60°C oven to evaporate the liquid to dryness. Finally, the sample impregnated with active components was calcined at 500° C. for 4 hours to obtain the desired catalyst.
[0022] Measure 10mL of catalyst plus 10mL of magnetic ring and place it in a stainless steel reaction tube with an inner diameter of 20mm, and reduce it under a hydrogen atmosphere or a carbon monoxide atmosphere at 180°C for 4 hours, and then introduce 100mL / min methyl nitrite when the temperature drops to room temperature. 150mL / min carbon monoxide and 150mL / min nitrogen, start to heat up to react, the reaction pressure is 0.05MPa, the reaction temperature is 135°C, and the average space-time yield of dimethyl oxalate is 950g / L·h. We f...
Embodiment 2
[0024] According to the loading capacity of 0.5wt% Pd, measure 15mL of 0.06mol / L palladium acetate in acetone solution, mix 10g α-Al 2 o 3 (specific surface area 3m 2 / g, particle size 2.5mm) soaked in this active ingredient solution for 12 hours, then transferred to a 60°C oven to evaporate the liquid to dryness. Finally, the sample impregnated with active components was calcined at 600° C. for 4 hours to obtain the desired catalyst.
[0025] Measure 10mL of the catalyst of Example 2 plus 10mL of the magnetic ring, evaluate the catalyst according to the evaluation conditions of Example 1, and the space-time yield of dimethyl oxalate is 1050g / L·h.
Embodiment 3
[0027] According to the loading capacity of 0.1wt% Pd, measure 15mL of 0.02mol / L palladium acetate in chloroform solution, mix 10g α-Al 2 o 3 (specific surface area 3m 2 / g, particle size 2.5mm) soaked in this active ingredient solution for 16 hours, then transferred to a 60°C oven to evaporate the liquid to dryness. Finally, the sample impregnated with active components was calcined at 400° C. for 4 hours to obtain the desired catalyst.
[0028] Measure 10mL of the catalyst of Example 3 plus 10mL of the magnetic ring, evaluate the catalyst according to the evaluation conditions of Example 1, and the space-time yield of dimethyl oxalate is 852g / L·h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com