Method for preparing novel mogrol derivatives from momordica grosvenori total saponins
A technology of mogrosides and mogrosanol, which is applied in the field of medicine, can solve the problems of low yield, difficult separation and purification, and difficult industrial production, and achieves the effect of low cost, simple synthesis method, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
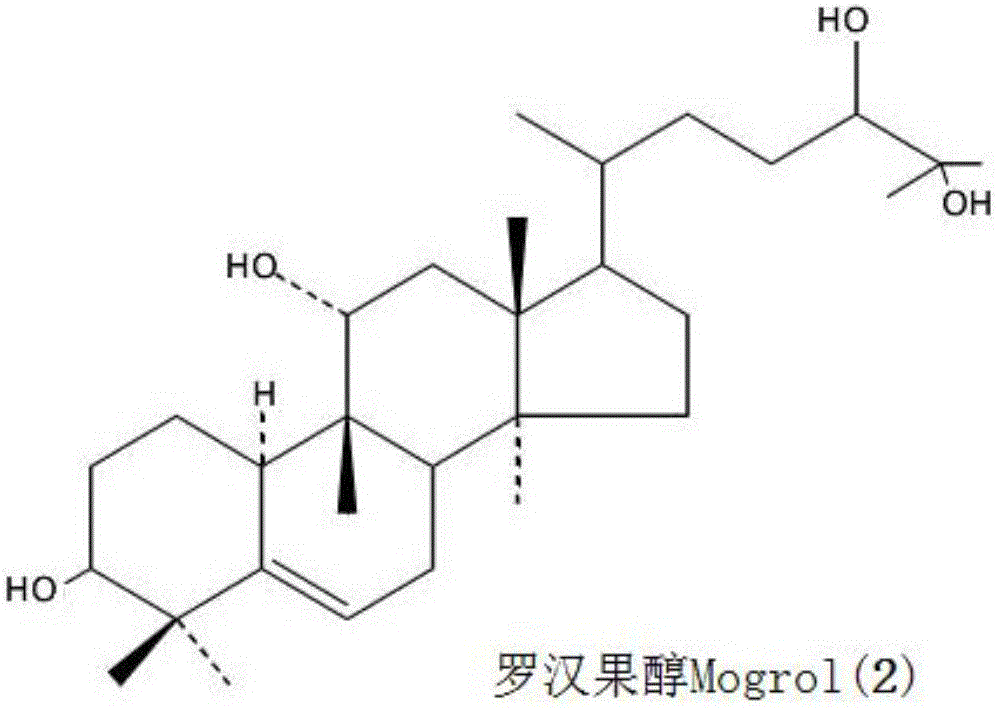
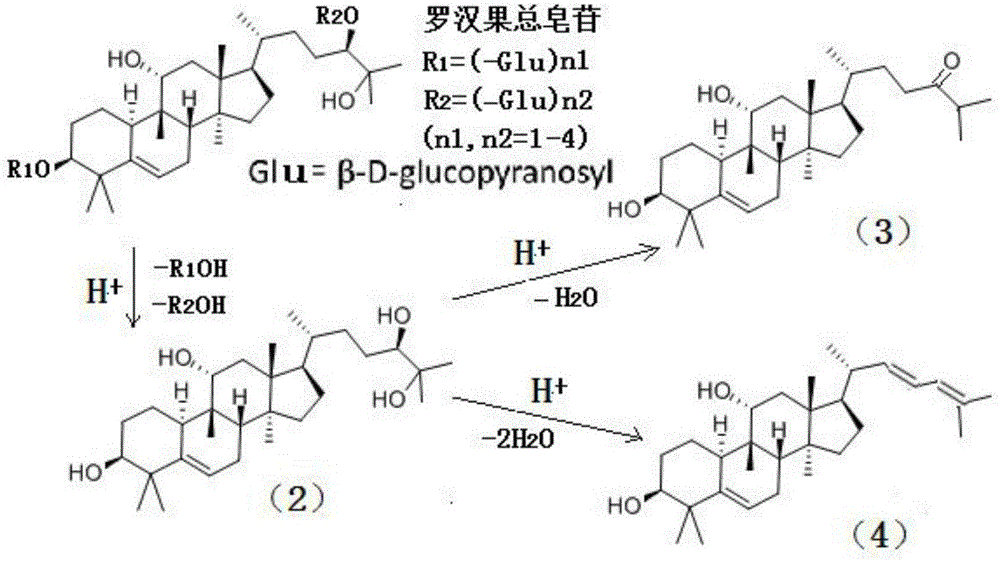
[0079] The present invention also provides a method for synthesizing the above composition, which comprises: dissolving the Monk Fruit extract containing total saponins of Monk Fruit in an acidic alcoholic aqueous solution and performing a heating reaction to obtain the composition.
[0080] The alcohol is at least one of methanol, ethanol, n-propanol, isopropanol, butanol; ethylene glycol, propylene glycol, glycerol; polyethylene glycol.
[0081] The preparation method of the acidic alcohol aqueous solution is: adjust the alcohol aqueous solution to be acidic with an acidic substance, preferably, the volume ratio of alcohol to water in the alcohol aqueous solution is 1:10-10:1; preferably 1:3 to 3:1;, more preferably 1:1.
[0082] Preferably, the acidic substance is an inorganic acid or an organic acid; further preferably, the inorganic acid is at least one of hydrochloric acid, sulfuric acid and phosphoric acid; the organic acid is formic acid, acetic acid, haloacetic acid, ...
Embodiment 1
[0111] Example 1: Preparation of Mogrosanol Derivatives Monomers (3) and (4) from the Extract of 98% Mogrosin Total Saponins
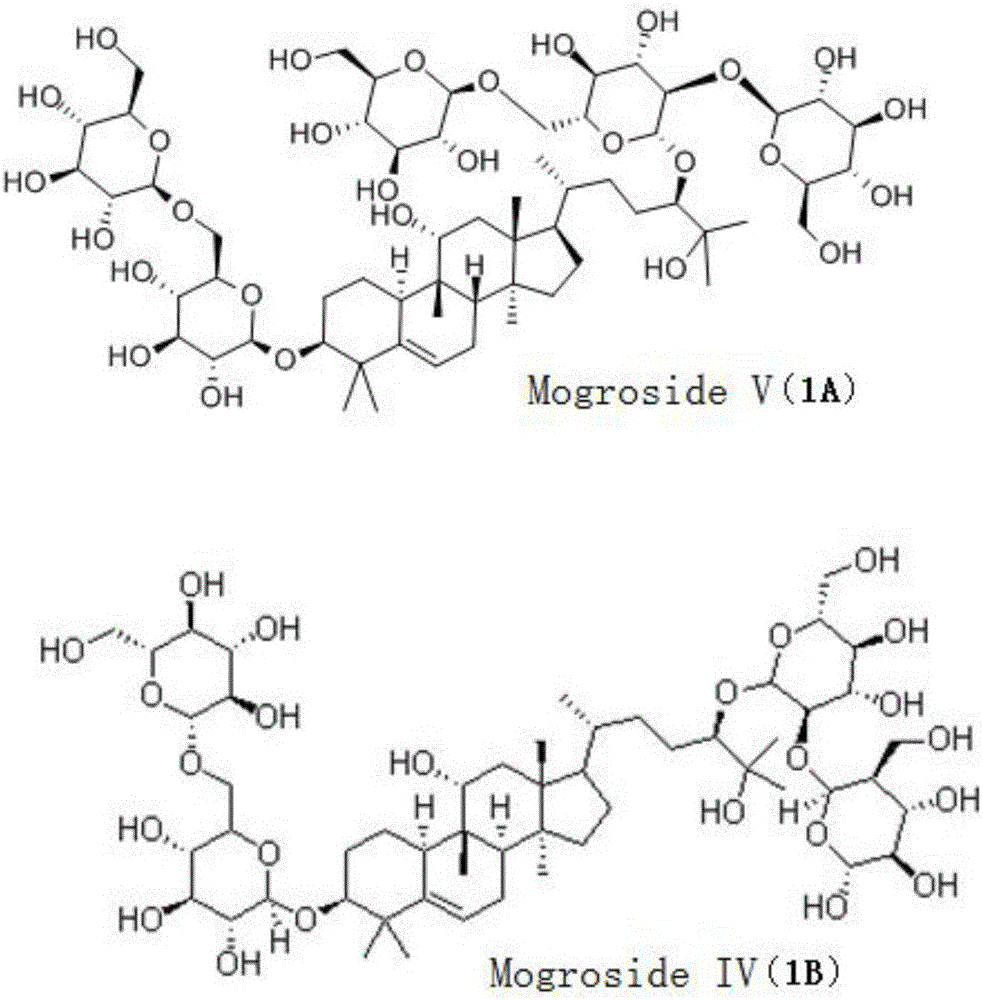
[0112] Add 50g of Luo Han Guo extract (98% of the total saponins of Luo Han Guo, containing 55% of Mogroside V, purchased from Guilin Laiyin Biotechnology Co., Ltd.) to 250 mL of ethanol aqueous solution (the volume ratio of ethanol and water is 1:1) (prepared with Hydrochloric acid adjusted to a pH value of 3.0), stirred and dissolved in the reactor, then heated to 120 ° C for 2 hours, cooled to room temperature 25 ° C, the reaction solution was neutralized to a pH value of 7 with 1M NaOH lye, and in Distill the ethanol as much as possible at 90-95°C, add 200mL of distilled water to the remaining concentrated solution, stir thoroughly, cool and let it stand, separate and discard the upper water layer to obtain a brown extract; use 100mL, 50mL, and 50mL of acetic acid respectively Extract the brown extract with ethyl ester for 3 times, combine the 3 ex...
Embodiment 2
[0114] Example 2: Preparation of Mogrosanol Derivative Monomers (3) and (4) from the Extract of 95% Mogrosin Total Saponins
[0115] Add 50g of Luo Han Guo extract (95% of the total saponins of Luo Han Guo, containing 45% of mogroside V, purchased from Guilin Laiyin Biotechnology Co., Ltd.) into 250 mL of methanol aqueous solution (the volume ratio of methanol and water is 3:2) (pre-used Sulfuric acid is adjusted to a pH value of 2.8), stirred and dissolved in the reactor, and then heated to 120°C for 2 hours, cooled to room temperature, and the reaction solution is neutralized with 1M KOH lye to a pH value of 7. Distill the methanol as much as possible at 95°C, add 200mL of distilled water to the remaining concentrated solution, stir thoroughly, cool and let it stand, separate and discard the upper water layer to obtain a brown extract; use 100mL, 50mL, and 50mL ethyl acetate respectively Extract the coffee-colored extract 3 times, combine the 3 extracts, concentrate with a r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com