1,3,4-thiadiazole derivatives, preparing method thereof and applications of the derivatives
A thiadiazole derivative, thiadiazole technology, applied in the direction of lubricating composition, petroleum industry, additives, etc., can solve the problems that are not involved, and achieve easy realization, high yield, good performance of anti-friction and extreme pressure resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
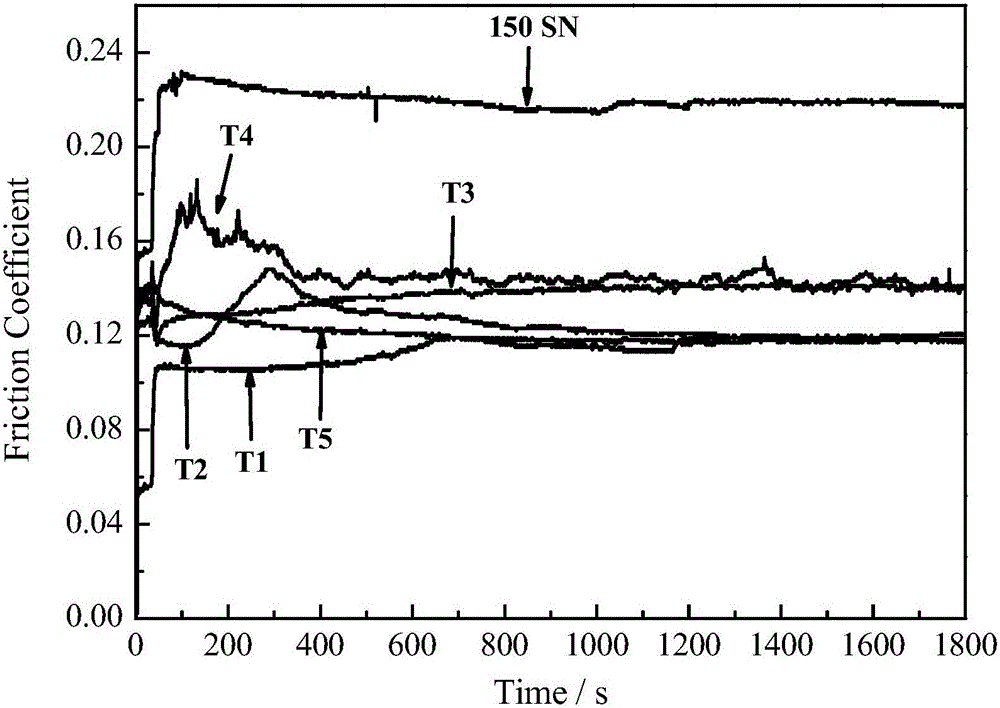
Examples
Embodiment 1
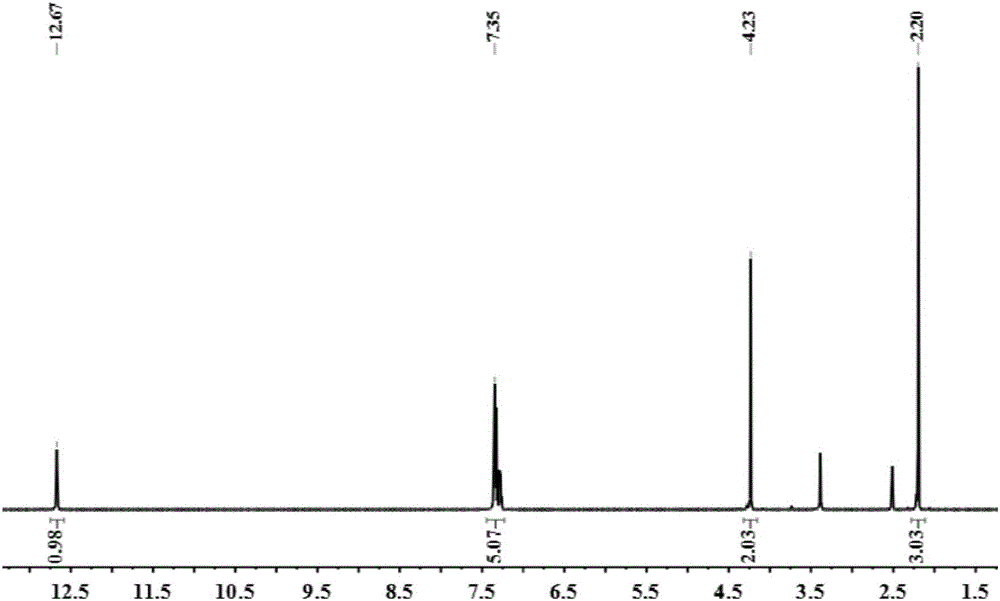
[0034] Example 1: Synthesis of compound 5-acetylamino-2-thio-n-octyl-1,3,4-thiadiazole (T1).
[0035] Add 1.32g (10mmol) 5-amino-2-mercapto-1,3,4-thiadiazole and 0.84g (15mmol) KOH into a 50ml round bottom flask, add 1ml deionized water, stir until all solids disappear, add 1.94g (10mmol) n-octane bromide, 10ml absolute ethanol. When a large amount of white solid appeared in the reaction system, the reaction was continued for another 2 h. After the reaction, 30ml of water was added, suction filtered, washed with water, and dried. Recrystallized from 95% ethanol to obtain 5-amino-2-thio-n-octyl-1,3,4-thiadiazole as a white solid. Add 1.68g (10mmol) of 5-amino-2-thio-n-octyl-1,3,4-thiadiazole, 0.94ml (10mmol) of acetic anhydride, and 1.2ml of glacial acetic acid into a 50ml round bottom flask at 70°C Heat and stir in a water bath, and continue to react for 1 h when a large amount of white solid appears in the reaction system. Cool, filter with suction, wash with water, dry, ...
Embodiment 2
[0038] Example 2: Synthesis of 5-acetylamino-2-thiobenzyl-1,3,4-thiadiazole (T2).
[0039] Add 1.76g (10mmol) of 5-acetamido-2-mercapto-1,3,4-thiadiazole and 1ml deionized water into a 50ml round bottom flask, add 0.84g (15mmol) KOH to the system and stir until solid All disappear, add 2.27g (10mmol) benzyl chloride, 10ml absolute ethanol. When a large amount of white solid appeared in the reaction system, the reaction was continued for another 30 minutes. After the reaction, add 30ml of water, filter with suction, wash with water, dry, and recrystallize from absolute ethanol to obtain 1.97g of white needle-like crystals of 5-acetylamino-2-thiobenzyl-1,3,4-thiadiazole, The yield was 74.09%, and the melting point was 169-170°C.
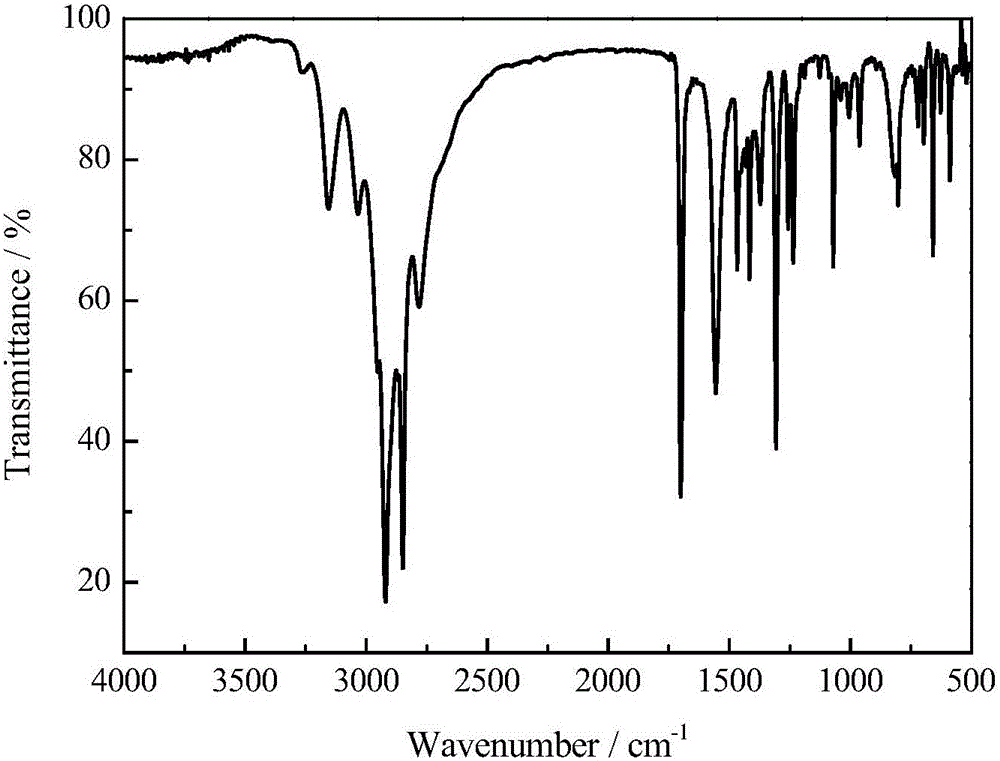
[0040] The structural characterization results of 5-acetylamino-2-thiobenyl-1,3,4-thiadiazole (T2), the data are as follows:
[0041] T2: IR (KBr, cm -1 )v: 3162.24, 3034.42, 2919.48, 2790.54, 1694.00, 1566.76, 1496.84, 1454.25, 1374.30, 1044.15, 769...
Embodiment 3
[0042] Example 3: Synthesis of the compound n-octyl 5-acetylamino-1,3,4-thiadiazole-2-thioglycolate (T3).
[0043] Take 1.17g (6.7mmol) of 5-acetylamino-2-mercapto-1,3,4-thiadiazole, 0.48g (8.6mmol) of KOH, and 5ml of deionized water in a 50mL round bottom flask, and stir until all the solids are After the disappearance, 5 mL of an ethanol solution containing 1.38 g (6.7 mmol) of n-octyl chloroacetate was slowly added dropwise under an ice-water bath. After 1 h of addition, the mixture was stirred at room temperature for 4 h. Suction filtration, washing with water, drying, and recrystallization from anhydrous methanol yielded 1.75 g of white needle-like crystals with a yield of 76.09% and a melting point of 121-123°C.
[0044] The structural characterization results of n-octyl 5-acetylamino-1,3,4-thiadiazole-2-thioglycolate (T3), the data are as follows:
[0045] T3: IR (KBr, cm -1 )v: 3160.94, 2926.65, 2852.40, 2791.81, 1740.76, 1687.94, 1567.20, 1467.97, 1377.21, 1159.80, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com