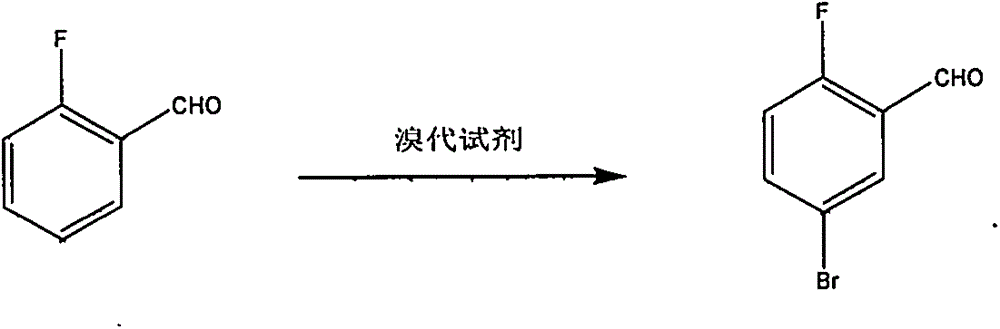
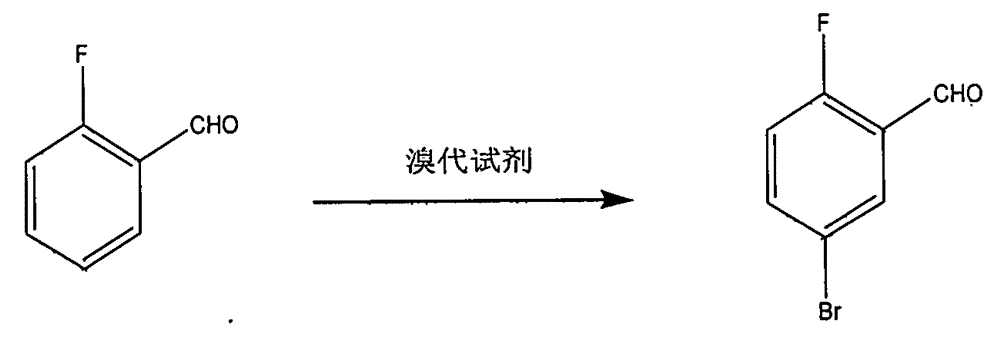
Preparation method of 2-fluoro-5-bromobenzaldehyde
A technology of bromobenzaldehyde and benzaldehyde, which is applied in the field of preparation of 2-fluoro-5 bromobenzaldehyde, can solve the problems of low applicability of synthesis route, increase of amplifying impurities, yield reduction, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Embodiment one: add 223ml concentrated sulfuric acid, 70g (0.56mol) o-fluorobenzaldehyde, 6g (0.045mol) anhydrous aluminum trichloride in the reactor that electric stirring is housed, thermometer and condensing tube, heat to 60 ℃ minute Add 105.5g (0.59mol) N-bromosuccinimide in batches, react for 3-8 hours, pour the system into ice water, extract with cyclohexane, wash the organic phase with water, wash with saturated brine, and anhydrous sodium sulfate Dry, filter, and concentrate to remove cyclohexane to obtain a brownish-red oil, which is collected by vacuum distillation at 63-65°C / 3mmHg fraction 91.6g, with a yield of 80% and a content of 98%.
Embodiment 2
[0009] Embodiment two: add dichloroethane and catalyzer zinc bromide 67g (0.3mol) in the reactor equipped with electric stirring, thermometer, dropping funnel and condenser, add o-fluorobenzene under stirring at 50 ℃~90 ℃ Add 62g (0.5mol) of formaldehyde, then add 80g (0.5mol) of bromine dropwise, and continue the reaction for 2-8 hours after the addition. The reaction solution was washed with water, pickled, dried and filtered over anhydrous sodium sulfate, concentrated to remove dichloroethane to obtain a brownish-red oil, and 76 g of 63-65°C / 3mmHg fraction was collected by rectification under reduced pressure, with a yield of 75% and a content of 99%.
Embodiment 3
[0010] Embodiment three: electric stirring is housed, thermometer, add 65% sulfuric acid aqueous solution 500ml, potassium bromate 167g (1mol) in the reactor of dropping funnel and condensing pipe, dropwise add o-fluorobenzaldehyde 124.1g (1mol), 90 ℃ of reactions After 2-3 hours, add 1000ml of water to the system, extract with methyl tert-butyl ether, wash the organic phase with an aqueous solution of sodium sulfite, add anhydrous sodium sulfate, dry, filter and concentrate to remove methyl tert-butyl ether to obtain a brownish-red oil, and decompress Rectification collects 63-65 ℃ / 3mmHg distillate 178g, yield 88%, content 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com