Quercetin-3-O-alpha-L-arabinose-(1->2)-alpha-L-rhamnoside prepared from red date leaves and application thereof
A technology of arabinose and rhamnoside, applied in the field of extraction and preparation of plant active ingredients, quercetin-3-O-α-L-arabinose--α-L-rhamnoside extraction, can solve the problem of poor yield. High, complex extraction and purification process, not the preparation process of flavonoid glycosides, etc., to achieve the effects of short preparation cycle, wide practical value, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Extraction of quercetin-3-O-α-L-arabinose-(1→2)-α-L-rhamnoside from jujube leaves
[0032] The specific extraction method steps for preparing quercetin-3-O-α-L-arabinose-(1→2)-α-L-rhamnoside from jujube leaves are as follows:
[0033] (1) Pretreatment of raw materials: Harvest fresh red jujube leaves without insect erosion, wash them with clean water, dry them at 45°C, crush them, and pass them through a 40-60 mesh sieve.
[0034] (2) Extraction and concentration: the pulverized red jujube leaves prepared in step (1) were extracted with 75% ethanol solution, and the ratio of solid to liquid was 1:10, that is, g / ml. After 2 h, filter, combine the filtrates for 4 times, and concentrate the extract under reduced pressure at low temperature to obtain the total flavonoid extract with a yield of 13.0%.
[0035] (3) Elution: Dissolve the total flavonoids extract obtained in step (2) with 4 times the volume of distilled water, and then put it on a macroporous resin ...
Embodiment 2
[0043] Example 2: Preparation of quercetin-3-O-α-L-arabinose-(1→2)-α-L-rhamnoside from jujube leaves
[0044] The specific extraction method steps for preparing quercetin-3-O-α-L-arabinose-(1→2)-α-L-rhamnoside from jujube leaves are as follows:
[0045] (1) Pretreatment of raw materials: Harvest fresh red jujube leaves in November without insect erosion, wash with clean water, dry at 45°C, crush, crush and pass through a 40-mesh sieve.
[0046] (2) Extraction and concentration: Weigh 500 g of crushed jujube leaves prepared in step (1) and extract with 5 L of 75% ethanol solution, reflux extraction at 60°C for 2 h each time, filter, and combine for 4 times The filtrate and the extract were concentrated under reduced pressure at low temperature to obtain the total flavonoid extract with a yield of 13.0%.
[0047] (3) Elution: Dissolve the total flavonoid extract obtained in step (2) with 500 ml of water and apply it to a macroporous resin column at a flow rate of 1 ml / min. Afte...
Embodiment 3
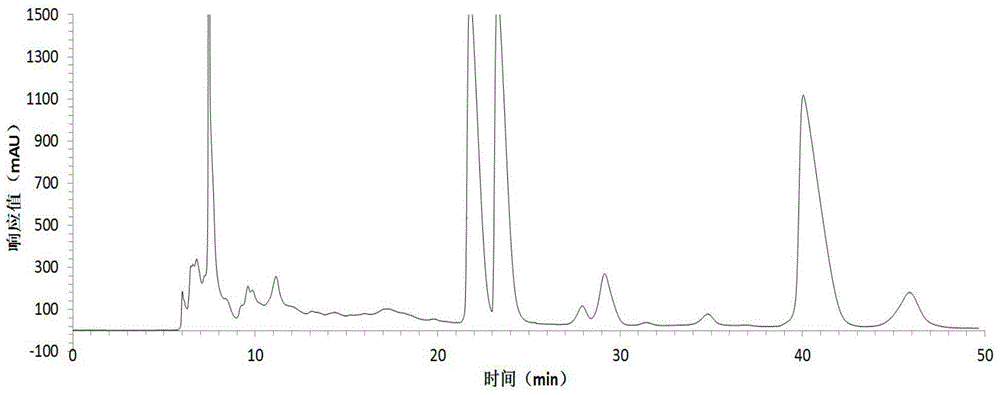
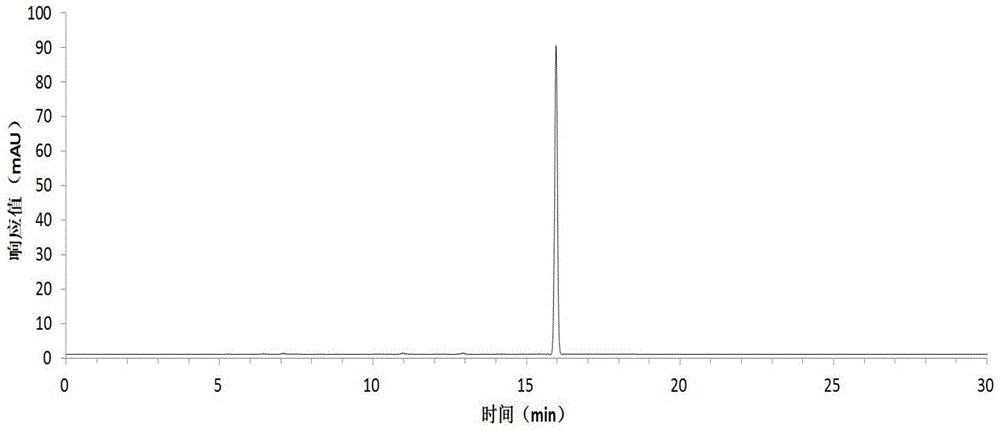
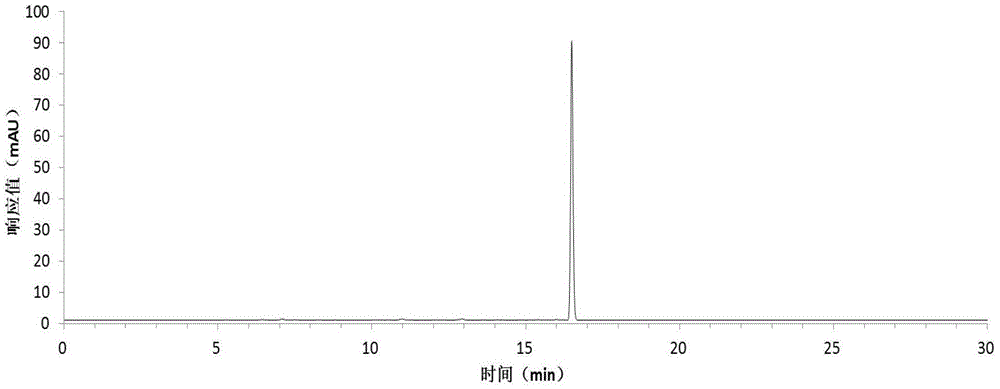
[0054] Harvest red jujube leaves, wash them with clean water, dry them at 45°C, grind the dried red jujube leaves with a pulverizer, extract the dried sample powder with 75% ethanol solution, concentrate under reduced pressure at low temperature, and obtain the total flavonoid extract with a yield of 13.0% . The total flavonoid extract was dissolved in an appropriate amount of water and applied to a macroporous resin column, eluted with 20%, 50%, and 95% ethanol solutions respectively, collected 50% of the eluate, and recovered the solvent to obtain crude flavonoid glycosides with a yield of 3.5%. The crude flavonoid glycosides were dissolved in 3 times the volume of 50% methanol and refined and purified by preparative HPLC, the eluent was detected by analytical HPLC, the same fractions with a purity greater than 98% were combined, the solvent was recovered, and quercetin was obtained respectively Quercetin-3-O-bacoside, quercetin-3-O-rutinoside and a rare flavonoid glycoside ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com