Method for preparing water-soluble mercury ion fluorescence probe on basis of rhodamine and application of water-soluble mercury ion fluorescence probe
A fluorescent probe, water-soluble technology, applied in the field of fluorescent probes, can solve problems such as limiting practical applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
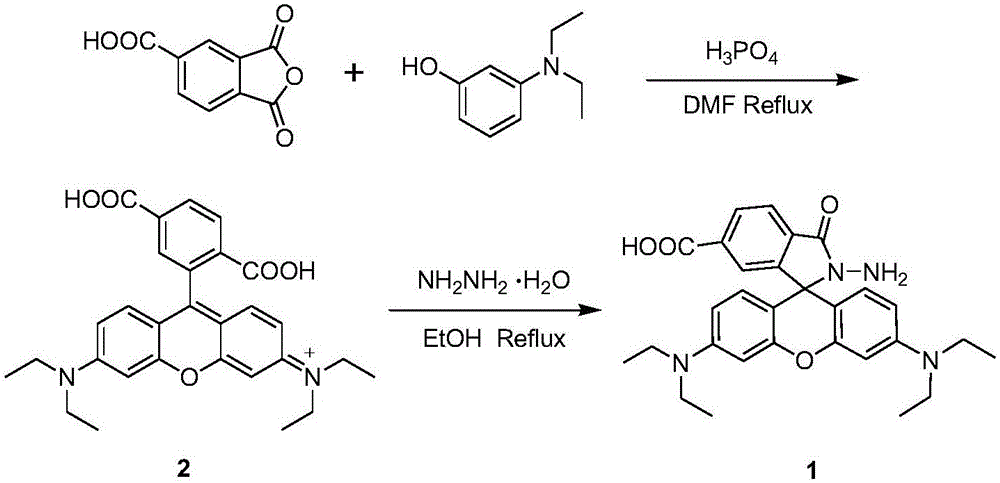
[0025] Synthesis of 5-carboxyrhodamine B (2): Add 1,2,4-trimellitic anhydride (1.92 g, 10 mmol) and 3-diethylamino to a 100 mL round bottom flask containing 50 mL N,N-dimethylformamide Phenol (3.30g, 20mmol), under the protection of nitrogen, magnetically stirred and refluxed for 6 hours, and the reaction was stopped; after the reaction mixture was cooled to room temperature, double distilled water (200mL) was added, and after fully stirring, it was carried out with dichloromethane (100mL×3) After extraction, the organic layers were combined and the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography with dichloromethane / methanol at 6:1 (volume ratio) to obtain red solid 2 (0.419g, yield: 8.6%). 1 H NMR (400MHz, CD 3 OD)δ8.19(d,J=8.0Hz,1H),8.08(d,J=8.0Hz,1H),7.80(s,1H),7.28(d,J=8.0Hz,2H),6.98(d ,J=8.0Hz,2H),6.89(s,2H),3.64(d,J=8.0Hz,8H),1.28(s,12H). 13 CNMR (100MHz, CD 3 OD)δ164.9, 160.4, 157.9, 134.3, 132.8, 132.6, 13...
Embodiment 2
[0028] Fluorescent probe 1 and Hg 2+ Solution preparation for action
[0029] Dissolve a certain amount of fluorescent probe in water to obtain a concentration of 1.0×10 -4 mol L -1 Probe stock solution. Dissolve a certain amount of mercury nitrate in water, pour it into a 500mL volumetric flask, add water to dilute to the mark, and obtain a concentration of 1.0×10 -3 mol L -1 Hg 2+ . will be 1.0×10 -3 mol L -1 Hg 2+ The aqueous solution was gradually diluted with double distilled water to obtain 1.0×10 -4 -1.0×10 -8 mol L -1 Hg 2+ aqueous solution. Mix 1.0 mL of probe stock solution and 1.0 mL of Hg 2+ The aqueous solution was added to a 10mL volumetric flask, and the buffer solution Tris-HNO 3 Constant volume, to obtain a concentration of 1.0 × 10 -5 mol L -1 fluorescent probe and 1.0 x 10 -4 -1.0×10 -8 mol L -1 Hg 2+ Mix the solutions to be tested.
Embodiment 3
[0031] Fluorescent probe 1 and Hg 2+ Determination of the fluorescence spectroscopic properties of the action
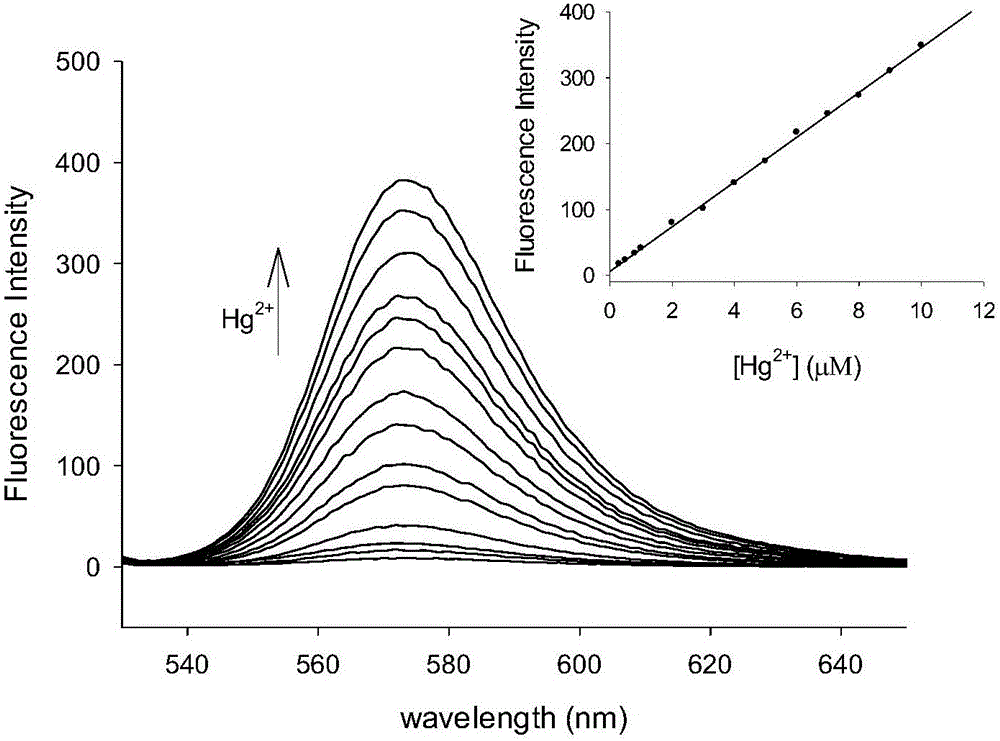
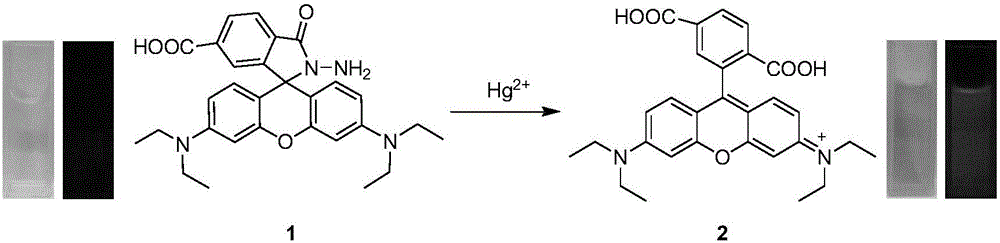
[0032] Tris-HNO at pH 7.0 3 The buffer solution was used as the solvent, and the fluorescent probe and Hg were measured with a Perkin Elmer LS 55 fluorescence spectrophotometer. 2+ The fluorescence spectrum of the action, the results are as follows figure 2 . The concentration of the fluorescent probe was 10 μM, Hg 2+ The concentration is 0, 0.3, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 20.0μM, the excitation wavelength is fixed at 520nm, the emission wavelength range is 530-650nm, the slit width It is 5nm / 5nm. Add Hg 2+ Before, the fluorescent probe was almost non-fluorescent, adding Hg 2+ After that, the emission peak of rhodamine appeared at 573nm, and with the Hg 2+ As the concentration increases, the fluorescence intensity of the probe molecule increases continuously. When 10.0 μM Hg is added 2+ When , the fluorescence intensity increase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com