Procaine hydrochloride micro-capsules and preparation method thereof
A technology of procaine hydrochloride and capsule material, which is applied in the field of procaine hydrochloride microcapsules and its preparation, and can solve the problems of inability to restrain the hydrolysis of procaine hydrochloride, poor drug use compliance, and toxic substances, etc. , to achieve the effects of improving bioavailability, improving drug compliance, and stabilizing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
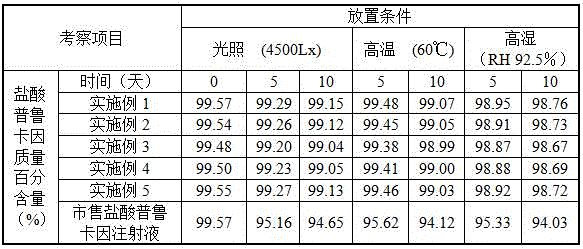
[0019] This embodiment provides a procaine hydrochloride microcapsule, which consists of procaine hydrochloride powder with a particle size of less than or equal to 30 microns and an ethyl cellulose capsule material comprising the procaine hydrochloride powder, and the ethyl cellulose The mass ratio of the base cellulose to the ethyl cellulose capsule material is 1:1.
[0020] The preparation method of the above-mentioned procaine hydrochloride microcapsules provided in this embodiment comprises the following steps: use absolute ethanol as a solvent to prepare a capsule material solution with an ethyl cellulose concentration of 1%, and then pulverize the procaine hydrochloride drug The particle size is less than or equal to 30 microns, suspended in the capsule material solution, and the mass ratio of procaine hydrochloride powder and capsule material is made into a uniformly dispersed suspension of 1: 1, and the spray drier is used at the import Under the condition that the te...
Embodiment 2
[0022] This embodiment provides a procaine hydrochloride microcapsule, which consists of procaine hydrochloride powder with a particle size of 10-30 microns and a sodium alginate capsule material comprising the procaine hydrochloride powder, and the seaweed The mass ratio of sodium acetate to the ethyl cellulose capsule material is 1:4.
[0023] The preparation method of the above-mentioned procaine hydrochloride microcapsules provided in this embodiment comprises the following steps: using absolute ethanol as a solvent to prepare a capsule material solution with a sodium alginate concentration of 3%, and then pulverizing the procaine hydrochloride drug to Particle diameter is 10~30 microns, is suspended in capsule material solution, makes procaine hydrochloride powder and capsule material mass ratio and is the uniformly dispersed suspension of 1: 4, adopts described spray drier at inlet temperature 150 DEG C, outlet temperature are 90 DEG C, feeding rate is 15 milliliters / min...
Embodiment 3
[0025] This embodiment provides a procaine hydrochloride microcapsule, which consists of procaine hydrochloride powder with a particle size of 1 to 25 microns and an acrylic resin RL100 capsule material comprising the procaine hydrochloride powder, and the acrylic acid The mass ratio of the resin RL100 to the ethyl cellulose capsule material is 1:6.
[0026] The preparation method of the above-mentioned procaine hydrochloride microcapsules provided in this embodiment comprises the following steps: use absolute ethanol as a solvent to prepare a capsule material solution in which the concentration of acrylic resin RL100 is 5%, and then pulverize the procaine hydrochloride drug to Particle diameter is 1~25 microns, is suspended in capsule material solution, and the evenly dispersed suspension that makes procaine hydrochloride powder and capsule material mass ratio is 1: 6, adopts described spray drier at inlet temperature 80 ℃, outlet temperature is 60 ℃, feed rate is 10 millilit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com