Method for preparing potassium sulfate from brine
A technology of potassium sulfate and brine, which is applied in the preparation of sulfate/bisulfate, tetrafluoroboric acid, boron halide compounds, etc., can solve the problems of high production cost, human health hazards, and high price, and achieve large industrial value and reduce Effects of pollution and resource saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
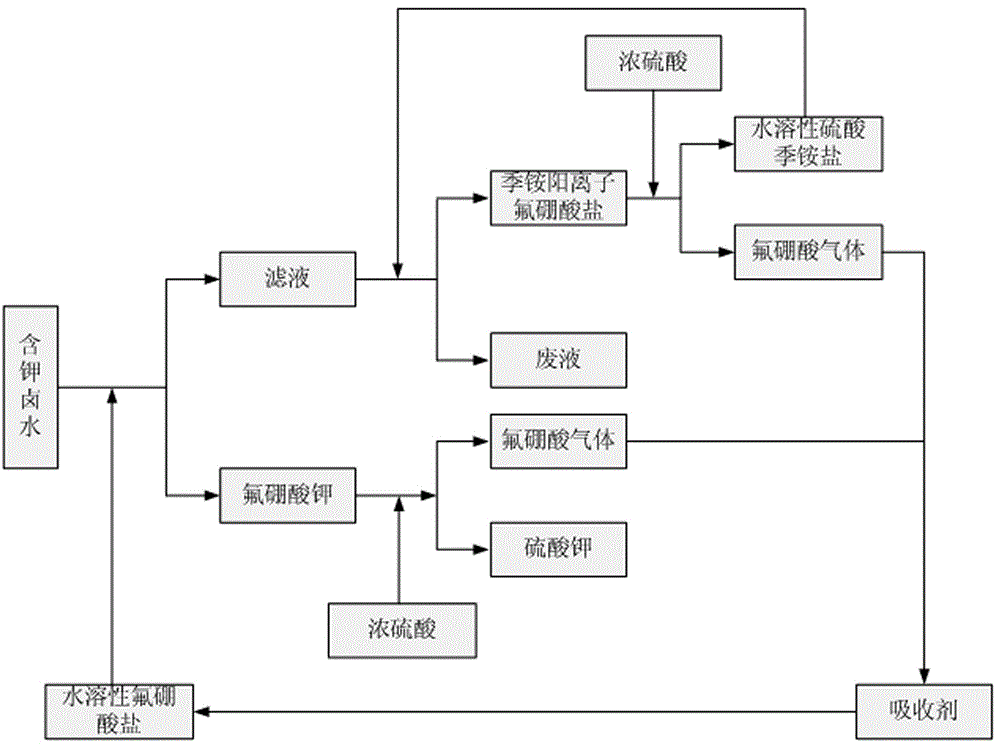
[0027] Take brine as the method for preparing potassium sulfate as raw material, the process flow chart is as figure 1 As shown, take 1.5L of the brine described in Table 1, add saturated sodium fluoroborate solution, the molar weight of sodium fluoroborate is 0.476mol, stir and react at room temperature for 20min, filter out the precipitate, and keep the filtrate; Dried and weighed, 52.73gKBF can be obtained 4 solid, the KBF 4 Mix the solid with 21.25g of concentrated sulfuric acid with a mass fraction of 98.3%, heat to 700°C, and collect fluoboric acid gas with a suspension containing 27.0g of sodium bicarbonate, stop the reaction after 50min, dry at 105°C and weigh The solid mass of potassium sulfate was 37.16g, the purity was 98.2%, and the recovery rate of potassium ions was 79.5%.
[0028] Add cetylpyridinium sulfate solution containing 0.0286mol to the filtrate obtained after extracting potassium, immediately generate cetylpyridinium fluoroborate precipitation, filter...
Embodiment 2
[0031] Take brine as the method for preparing potassium sulfate as raw material, the process flow chart is as figure 1 As shown, get 1.5L of brine described in Table 1, add the saturated sodium fluoroborate solution recovered in Example 1, the molar weight of sodium fluoroborate is 0.467mol, after stirring and reacting at room temperature for 20min, filter out the precipitate, and keep the filtrate; The precipitate is dried at 105°C and weighed to obtain 51.78gKBF 4 solid, the KBF 4 Mix the solid with 21.05g of concentrated sulfuric acid with a mass fraction of 98.3%, heat to 700°C, and collect fluoboric acid gas with a suspension containing 25.0g of sodium bicarbonate, stop the reaction after 50min, dry at 105°C and weigh The solid mass of potassium sulfate is 36.45g, the purity is 98.3%, and the recovery rate of potassium ions is 78.1%.
[0032]Add the solution containing 0.0282mol cetylpyridinium sulfate recovered in embodiment 1 to the filtrate obtained after extracting ...
Embodiment 3
[0035] Take brine as the method for preparing potassium sulfate as raw material, the process flow chart is as figure 1 As shown, take 1L of the brine described in Table 1, add saturated ammonium fluoroborate solution, the molar weight of ammonium fluoroborate is 0.380mol, stir and react at room temperature for 20min, filter out the precipitate, and keep the filtrate; dry the precipitate at 105°C , weighing, 39.54gKBF can be obtained 4 solid, the KBF 4 The solid is mixed with 15.94g of concentrated sulfuric acid with a mass fraction of 98.3%, heated to 600°C, and the fluoroboric acid gas is collected with a solution containing 30.0g of ammonium bicarbonate. After 90 minutes, the reaction is stopped, and potassium sulfate is weighed after drying at 105°C. The solid mass was 28.15g, the purity was 97.2%, and the recovery rate of potassium ions was 89.4%.
[0036] Add dodecyltrimethylammonium sulfate solution to the filtrate obtained after extracting potassium, immediately gener...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com