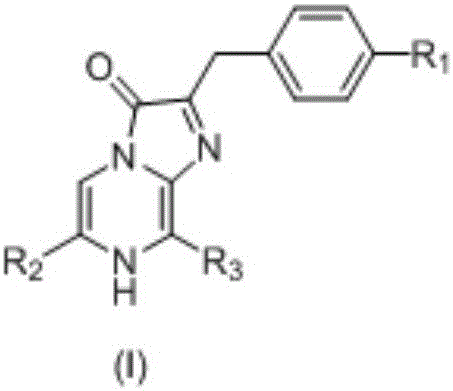
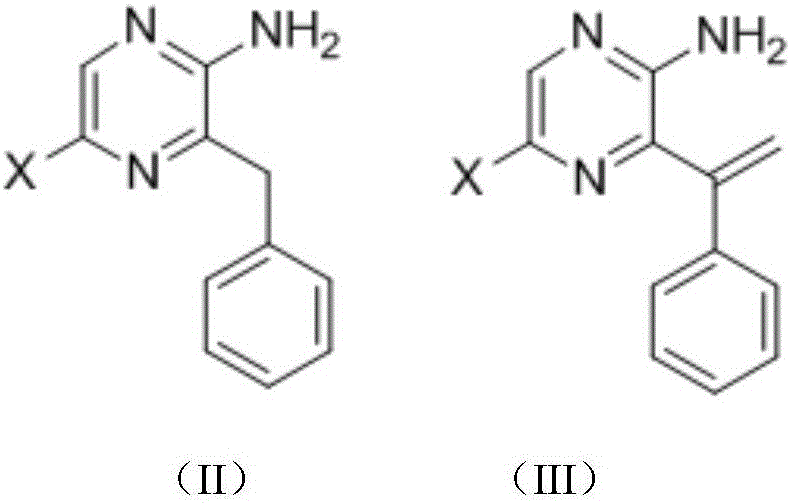
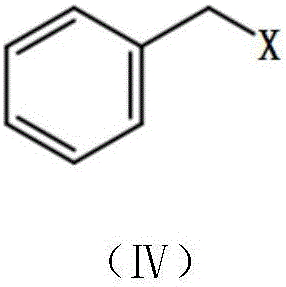
Coelenterazine analog and preparing method and application thereof
An analogue, coelenterazine technology, applied in the field of coelenterazine analogues and its preparation, can solve problems such as the influence of bioluminescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0146] [Example 1: 2-(phenylmethyl)-6-(4-fluorophenyl)-8-benzyl-imidazo[1,2-a]pyrazin-3-(7H)-one (B1) Preparation]
[0147] Preparation of intermediate 2-amino-5-bromo-3-phenylmethyl-pyrazine (1-1)
[0148] Under nitrogen protection, zinc powder (235 mg, 3.6 mmol) and iodine particles (12 mg) were mixed, DMF was added, and stirred at room temperature until the color of iodine disappeared. Then benzyl bromide (0.57mg, 3mmol) was added, moved to 80°C under reflux and stirred for about 3 hours. After the reaction solution was allowed to cool to room temperature, 2-amino-3,5-dibromopyrazine (506 mg, 2 mmol) and triphenylphosphine palladium dichloride (70 mg, 0.1 mmol) were added. React at room temperature for 24 hours. The reaction solution was filtered with diatomaceous earth, extracted with ethyl acetate, the ethyl acetate phase was washed with saturated sodium chloride solution, the ethyl acetate phase was dried with anhydrous sodium sulfate, filtered, and purified by 200-30...
example 2
[0155] [Example 2: 2-(phenylmethyl)-6-(3-fluoro-4-aminophenyl)-8-benzyl-imidazo[1,2-a]pyrazine-3-(7H)- Preparation of ketone (B2)]
[0156] Preparation of intermediate 2-amino-5-(4-amino-3-fluoro-phenyl)-3-benzyl-pyrazine
[0157] Under nitrogen protection, the intermediate 2-amino-5-bromo-3-benzyl-pyrazine (200mg, 0.756mmol), bisbenzonitrile palladium dichloride (II) (20mg, 0.050mmol), [1, 4-bis(diphenylphosphino)butane]palladium(II) dichloride (30 mg, 0.050 mmol) was dissolved in 8 mL of toluene, then 3-fluoro-4-amino-phenylboronic acid pinacol ester (240 mg , 1.058mmol) and aqueous potassium carbonate (2M, 0.6mL). Stir at reflux at 109°C for 10 hours. After stopping the reaction, the reaction solution was filtered, the filtrate was concentrated, ethyl acetate and saturated sodium chloride solution were added, and after extraction, the ethyl acetate layer was dried with anhydrous sodium sulfate, filtered, and 200-300 mesh silica gel column chromatography to obtain 133 mg ...
example 3
[0160] [Example 3: 2-(phenylmethyl)-6-(4-hydroxymethylphenyl)-8-benzyl-imidazo[1,2-a]pyrazin-3-(7H)-one ( Preparation of B3)]
[0161] Preparation of intermediate 2-amino-5(4-hydroxymethylphenyl)-3-benzyl-pyrazine
[0162] Under nitrogen protection, the intermediate 2-amino-5-bromo-3-benzyl-pyrazine (100mg, 0.378mmol), bisbenzonitrile palladium dichloride (II) (10mg, 0.026mmol), [1, 4-bis(diphenylphosphino)butane]palladium(II) dichloride (20 mg, 0.033 mmol) was dissolved in 6 mL of toluene, then 4-hydroxymethylphenylboronic acid (85 mg, 0.559 mmol) and sodium carbonate were added Aqueous solution (1M, 0.4 mL). Stir at reflux at 109°C for 10 hours. After stopping the reaction, the reaction solution was filtered, the filtrate was concentrated, ethyl acetate and saturated sodium chloride solution were added, and after extraction, the ethyl acetate layer was dried with anhydrous sodium sulfate, filtered, and 70 mg of a yellow solid was obtained by 200-300 mesh silica gel column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com