Breviscapine-panax notoginseng saponins double-pharmaceutical nano-composite particle and preparation method and application thereof
A technology of nanocomposite particles and Panax notoginseng saponins, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., to achieve the effects of low toxicity, improved solubility, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The embodiments of the present invention will be described in detail below in conjunction with specific examples, which are only used to illustrate the present invention, and should not be regarded as limiting the scope of rights of the present invention. For those not marked with specific conditions in the implementation plan, follow the conventional conditions or the conditions suggested by the manufacturer. The reagents or instruments used were not marked with manufacturers, and they were all conventional products that could be purchased from the market. Example 1: Preparation of breviscapine-notoginseng saponins nanocrystal suspension in different average particle size ranges (100nm, 200nm, 500nm, 800nm, 900nm)
[0032] Weigh 0.05g of Panax notoginseng saponins and add 100ml of water to dissolve to obtain a stabilizer solution; then, 0.5g of scutellarin is dispersed in the stabilizer solution, and sheared by high-speed shear emulsification, the rotating speed is: 13...
Embodiment 2
[0038] Example 2: Determination of Saturation Solubility of Breviscapine Nanocrystal Suspensions with Different Particle Sizes
[0039] Dissolution was determined by the paddle method, using 900 mL of pH 7.4 phosphate buffer solution as the dissolution medium, and the speed was 100 r min -1 , the temperature of the water bath is 37 ° C, and the average particle diameter prepared in the above example 1 is 10ml each of 500nm, 600nm, 700nm, 800nm, and 900nm, and the suspension is placed in the dissolution cup for 0.5min and 1min. After 1.5min, 4min, 6min, 10min, 20min, and 30min, 1 mL of sample was taken, filtered through a 0.22 μm microporous membrane, and determined by HPLC. According to the scutellarin standard curve, A=0.0504C+0.0903(R 2 =0.9994), the linear range is 0.36~20 μg / ml, and its dissolution rate is calculated, and its content is shown in Table 1.
[0040] Table 1 Comparison of the dissolution rate of breviscapine nanocrystal suspensions in different particle size ...
Embodiment 3
[0043] Example 3: Preparation of breviscapine-total notoginseng saponins nanocomposite particles
[0044] Experimental method: take the average particle diameter obtained according to the experimental method of Example 1 as 200nm
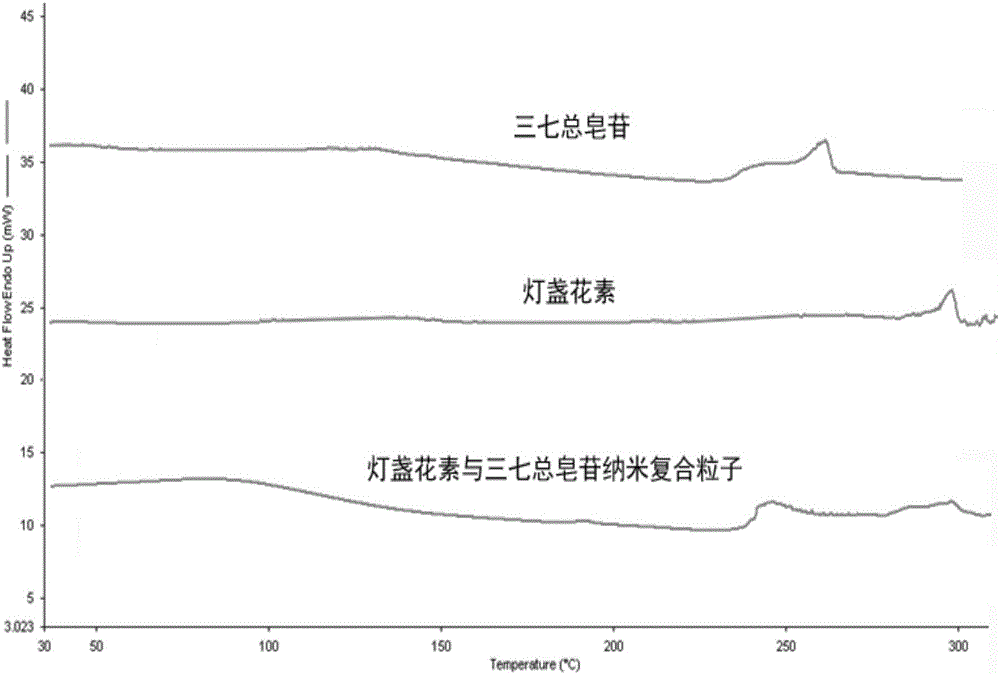
[0045] Add 1.0g of lactose (protective agent) to disperse and dissolve the sample of the nanocrystal suspension, and then use spray drying and rapid curing technology. The spray drying hot air temperature is 120°C, the air outlet temperature is 60°C, and the peristaltic speed of the pump is 5ml / min. After drying and curing, nanocomposite particle powder of scutellarin and Panax notoginseng saponins can be obtained.
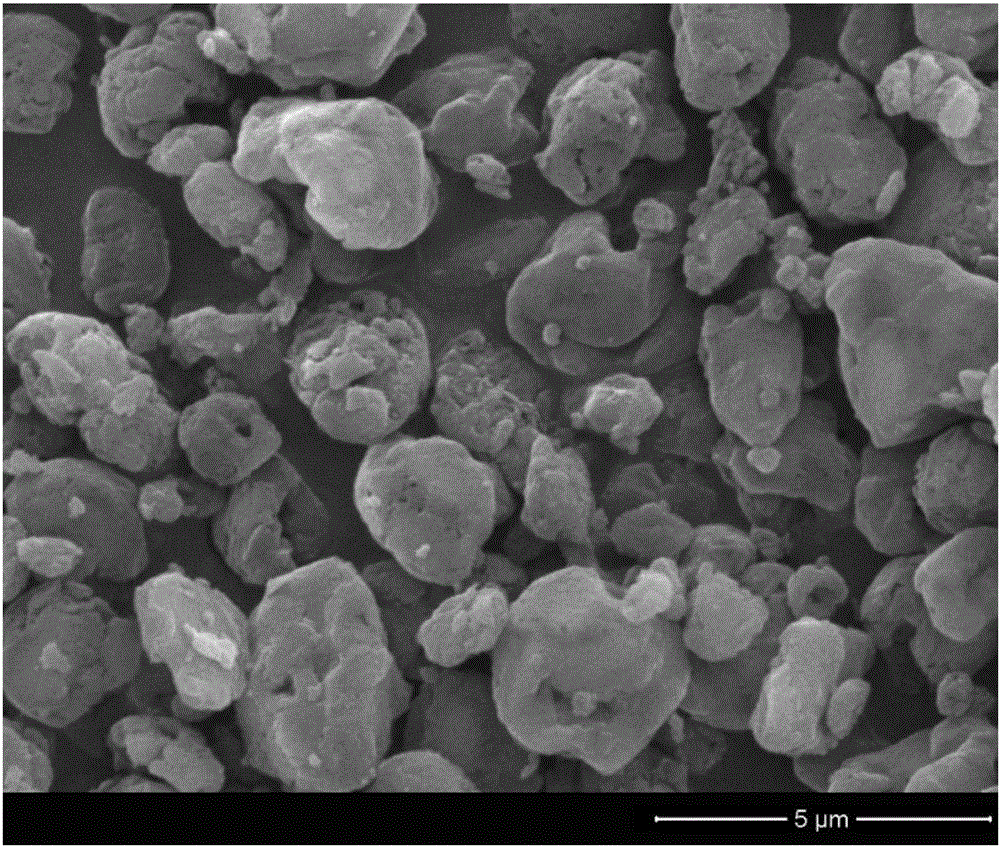
[0046] Add breviscapine and panax notoginseng saponins to redissolve and disperse in an appropriate amount of water, put a drop on a copper grid covered with a carbon film, and dry at room temperature. The morphology of scutellarin nanoparticles was observed by transmission electron microscope, and the results are shown in the attached...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com