Preparation method of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate
A technology of pentanediol diisobutyrate and trimethyl, which is applied in the field of preparation of plasticizer, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, can Solve the problems of difficult acquisition of raw materials, complex process, and production limitations, and achieve the effects of convenient production efficiency, simple process flow, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
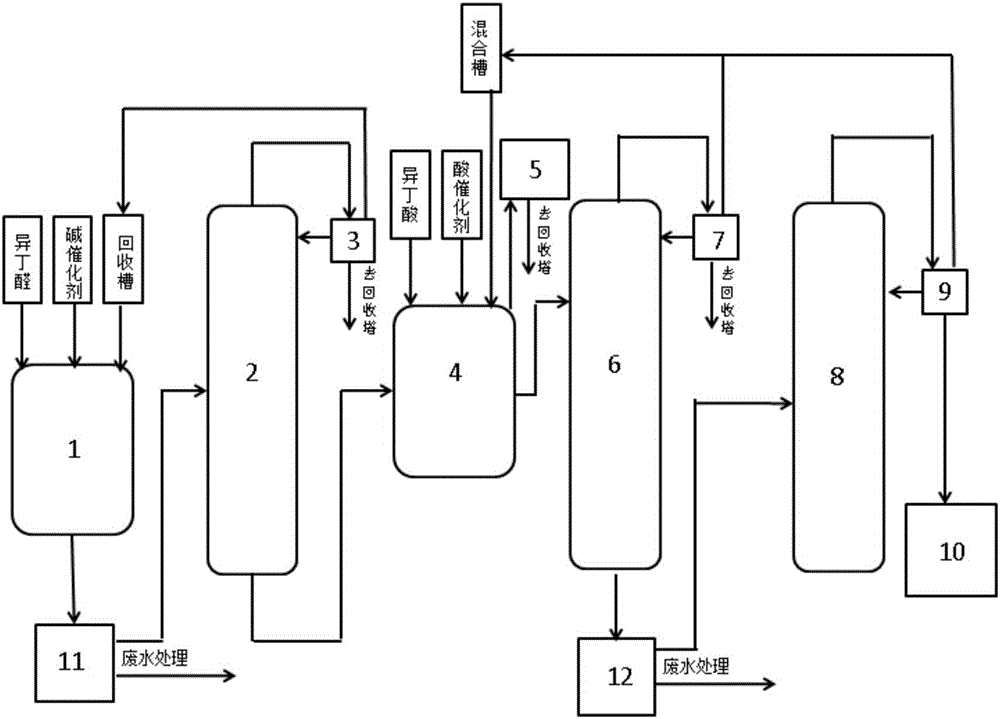
[0029] like figure 1 As shown, the present invention discloses a preparation method of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, comprising the following steps:
[0030] A. Using isobutyraldehyde as raw material, add alkali catalyst sodium hydroxide, potassium hydroxide, magnesium hydroxide, barium hydroxide, calcium hydroxide, and zinc hydroxide mixture, and control the alkali catalyst to be 5% of the mass of isobutyraldehyde. Butyraldehyde and alkali catalyst are added to the first reaction kettle to carry out a first-stage reaction, and the reaction temperature of the first-stage reaction is controlled to be 45°C for 2 hours; then the temperature is raised to carry out the second-stage reaction, and the reaction temperature of the second-stage reaction is controlled to be 75°C for 2.5 hours to generate 2, The crude product of 2,4-trimethyl-1,3-pentanediol, then pump the crude product of 2,2,4-trimethyl-1,3-pentanediol through the bottom of the first reaction kettle In...
Embodiment 2
[0038] like figure 1 As shown, the present invention discloses a preparation method of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, comprising the following steps:
[0039] A. Use isobutyraldehyde as raw material, add alkali catalyst magnesium alkoxide, calcium alkoxide, sodium alkoxide mixture, control the alkali catalyst to be 3% of the mass of isobutyraldehyde, add isobutyraldehyde and alkali catalyst into the first reaction kettle for a stage of reaction, Control the reaction temperature of the first-stage reaction at 40°C for 3 hours; then raise the temperature to carry out the second-stage reaction, control the reaction temperature of the second-stage reaction at 80°C for 2 hours to generate crude 2,2,4-trimethyl-1,3-pentanediol product, then pump the crude product of 2,2,4-trimethyl-1,3-pentanediol into the acid tower through the bottom of the first reaction kettle, and let it stand after acid recoil, neutralization and extraction Phase separation, the organic phase ...
Embodiment 3
[0047] like figure 1 As shown, the present invention discloses a preparation method of 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, comprising the following steps:
[0048] A. Take isobutyraldehyde as raw material, add alkali catalyst barium alkoxide, control the alkali catalyst to be 1% of the isobutyraldehyde quality, add isobutyraldehyde and alkali catalyst in the first reaction kettle to carry out one-stage reaction, control the reaction temperature of one-stage reaction to be React at 50°C for 2 hours; then raise the temperature to carry out the second-stage reaction, control the reaction temperature of the second-stage reaction at 70°C for 2.5 hours to generate 2,2,4-trimethyl-1,3-pentanediol crude product, then put 2, The crude product of 2,4-trimethyl-1,3-pentanediol is pumped into the acid tower through the bottom of the first reaction kettle. The pump is pumped into the first rectification tower, and the brine-containing phase is pumped into the wastewater treatme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com