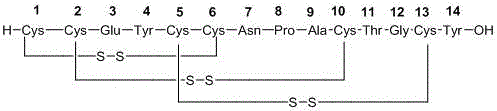
Method for preparing linaclotide
A technology of linaclotide and crude peptide, which is applied in the field of linaclotide preparation, can solve the problems of low yield, high cost of raw materials, cumbersome synthesis steps, etc., and achieve high yield, avoid mismatch and reduce cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of Linaclotide linear peptide protection resin
[0030] (1) Preparation of HMP-AM resin with substitution degree of 0.2~0.6 mmol / g
[0031] 445 g (200 mmol) of HMP-AM resin was prepared by the Fmoc protection method, and the degree of substitution measured with an ultraviolet absorbance photometer was 0.45 mmol / g.
[0032] (2) Preparation of Linaclotide linear peptide resin
[0033] Using an automatic peptide synthesizer, first react the Fmoc-Tyr(tBu)-resin from the previous step in a 20% mass percentage Pip / DMF solution for 20 minutes, remove the protective group Fmoc, and then automatically wash with DMF / DCM solution 2 times to prepare for the next amino acid link.
[0034] Use the program set up by the peptide synthesizer to couple amino acids one by one according to the peptide sequence. The specific reaction process is as follows: Fmoc-AA-OH (3 eq, 600 mmol) and HOBt (3 eq, 600 mmol) are dissolved in DMF (2.0 L), and then DIC (3 eq, 600 mmol) is adde...
Embodiment 2
[0035] Example 2: Preparation of Linaclotide Linear Peptide
[0036] Under the protection of nitrogen, linaclotide linear peptide resin is cleaved in a TFA / TIS / EDT / water volume ratio (94:2:2:2) solution. The specific operation is as follows: mix the linaclotide resin from the previous step with the TFA / TIS / EDT / water solution (5-10 mL / g), react for 4 hours, filter to remove the cracked resin, and then wash with TFA solution 2 all over. Combine the filtrate, evaporate the filtrate with a rotary evaporator, and then add cold ether (5-10 mL / g). The solid precipitates out. Filter and wash with cold ether 3 times to obtain a linear peptide of linaclotide, a white solid of 251.97 g, purity : 62%, yield: 50.9%.
Embodiment 3
[0037] Example 3: Preparation of linaclotide crude peptide
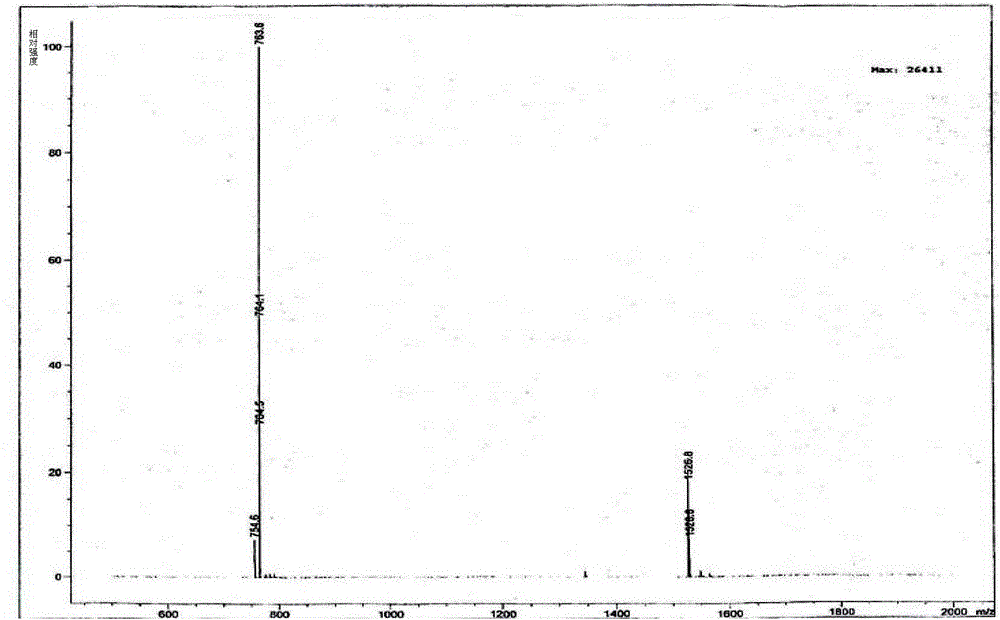
[0038] Linaclotide linear peptide (60.0 g) was dissolved in a mixed aqueous solution (25 L) of 1% by mass ammonia water and 30% by mass acetonitrile, and then added 2% by mass DMSO solution, During the reaction, the pH was adjusted to 9-10 with ammonia water, stirred at room temperature for 24 hours, and the progress of the reaction was monitored by LC-MS. Repeat the above experimental process to oxidize all linear peptides of linaclotide to linaclotide. 252.0 g of linaclotide was obtained, purity: 50.1%, yield: 41.1%.
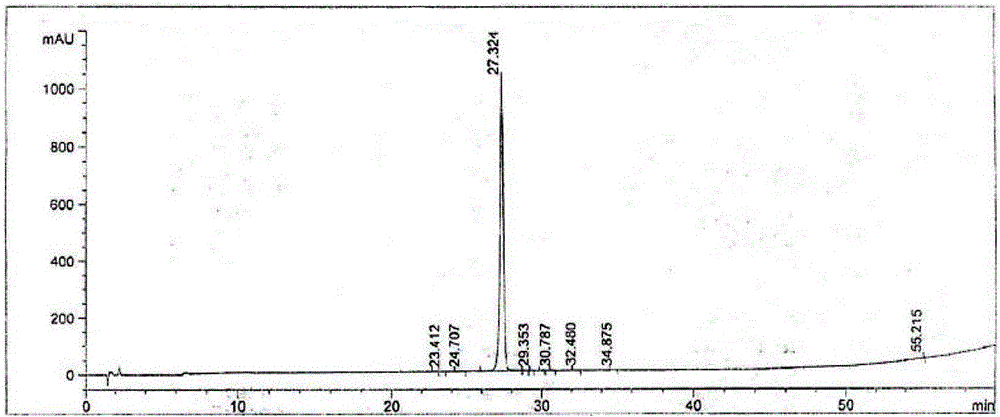
[0039] Note: The monitoring and analysis conditions of LC-MS are as follows
[0040] Chromatographic conditions: Agilent 1290; C18 column (1.7µm, 150x3mm); detection wavelength: λ=214nm
[0041] Mobile phase A: 0.1% mass percent TFA aqueous solution;
[0042] Mobile phase B: 0.1% mass percent acetonitrile solution of TFA chromatography;
[0043] Gradient elution: mobile phase A: mobile phase B mass percentag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com