Surface coating modification method of LiNi0.5Mn1.5O4 cathode material
A technology of lithium nickel manganese oxide and positive electrode materials, which is applied in the field of surface coating modification of lithium nickel manganese oxide positive electrode materials, can solve problems such as being susceptible to corrosion and reduced cycle performance, and achieve improved cycle performance, corrosion inhibition, and good lithium The effect of ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
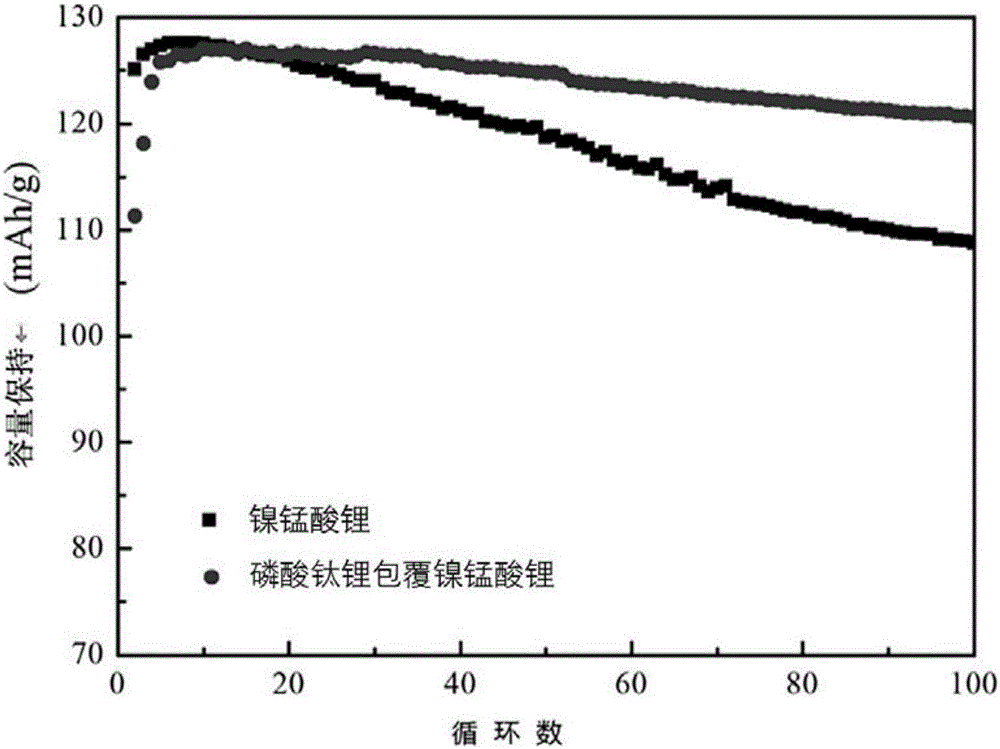
Image
Examples
Embodiment 1
[0037] (1) The preparation process of lithium nickel manganese oxide required for the implementation of coating in the present invention is as follows: respectively weigh 1.25 parts by weight of nickel acetate and 3.68 parts by weight of manganese acetate, add 80 mL of deionized water to dissolve completely, and drop in the concentration within 1 hour. 11mL of 1mol / L LiOH solution, add an appropriate amount of ammonia water to adjust the pH value to 11-12, heat and concentrate the co-precipitation solution at 80°C until viscous, transfer it to a blast drying oven for 6 hours at 100°C, and dry After grinding, place the product in a muffle furnace for pre-calcination at 500°C in an air atmosphere for 6 hours, grind the pre-fired product fully, press it into tablets under a pressure of 5 MPa, place the sample sheet in a high-temperature reaction furnace at 700°C for 3 hours, and heat up Heat to 900°C and keep it warm for 6 hours, lower the temperature to 650°C and keep it warm for...
Embodiment 2
[0042] (1) Dissolve 0.0129g of lithium acetate and 0.105g of n-butyl titanate in absolute ethanol solution, then add 2g of lithium nickel manganese oxide, heat up with stirring, react at 80°C for 2 hours, then evaporate the solvent until it evaporates to dryness , Lithium nickel manganese oxide coated with lithium acetate-titanium dioxide was obtained.
[0043] (2) According to the mass ratio of 3:97, 0.0534g of ammonium dihydrogen phosphate and the lithium acetate-titanium dioxide-coated lithium nickel manganese oxide prepared in step 1 were mixed and dispersed in deionized water, and the percentage concentration range was 20%. Stir and heat up, react at 80° C. for 5 hours, and then dry the solution to obtain lithium acetate-titanium dioxide-ammonium dihydrogen phosphate composite-coated lithium nickel manganese oxide.
[0044] (3) Put the lithium acetate-titanium dioxide-ammonium dihydrogen phosphate compound-coated lithium nickel manganese oxide obtained in step 2 into an a...
Embodiment 3
[0048] (1) Dissolve 0.065g of lithium acetate and 0.175g of n-butyl titanate in absolute ethanol solution, then add 2g of lithium nickel manganese oxide, heat up under stirring, react at 70°C for 3 hours, then evaporate the solvent until evaporated to dryness , Lithium nickel manganese oxide coated with lithium acetate-titanium dioxide was obtained.
[0049] (2) According to the mass ratio of 5:95, 0.089 g of ammonium dihydrogen phosphate and the lithium acetate-titanium dioxide-coated lithium nickel manganese oxide prepared in step 1 were mixed and dispersed in deionized water, and the percentage concentration range was 15%. Stir and heat up, react at 90° C. for 4 hours, and then dry the solution to obtain lithium acetate-titanium dioxide-ammonium dihydrogen phosphate composite-coated lithium nickel manganese oxide.
[0050] (3) Put the lithium acetate-titanium dioxide-ammonium dihydrogen phosphate compound-coated lithium nickel manganese oxide obtained in step 2 into an alum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com