Polysaccharide medicine loading tissue adhesion film and preparing method thereof
A film-adhering and drug-carrying technology, applied in the field of biomedicine, can solve the problems of inability to achieve clinical therapeutic effects, poor targeting, and large dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
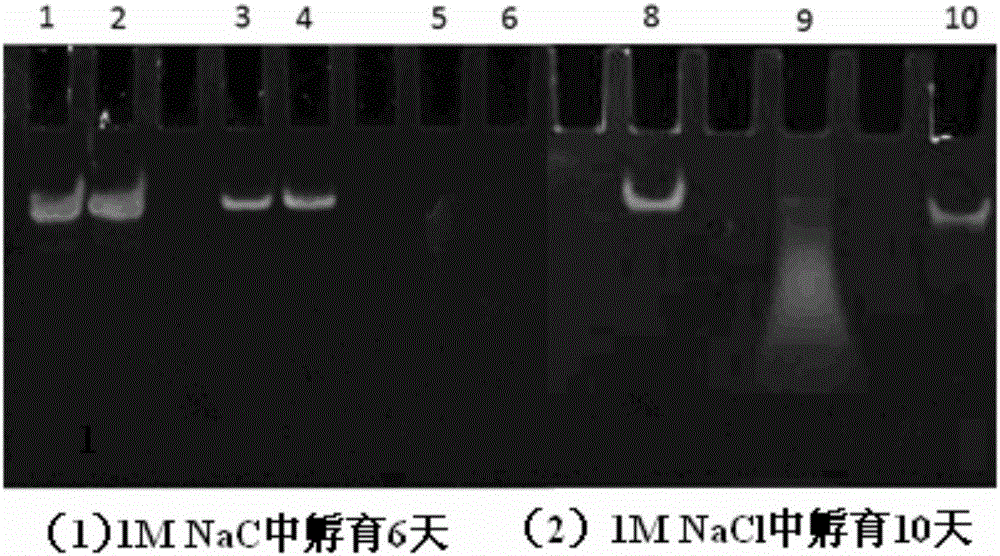
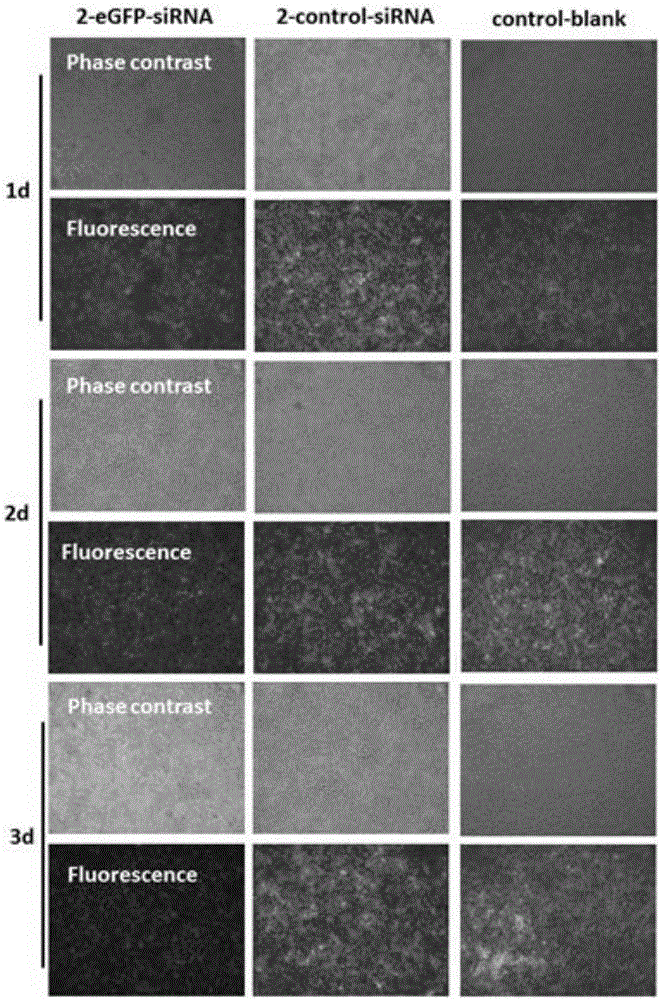
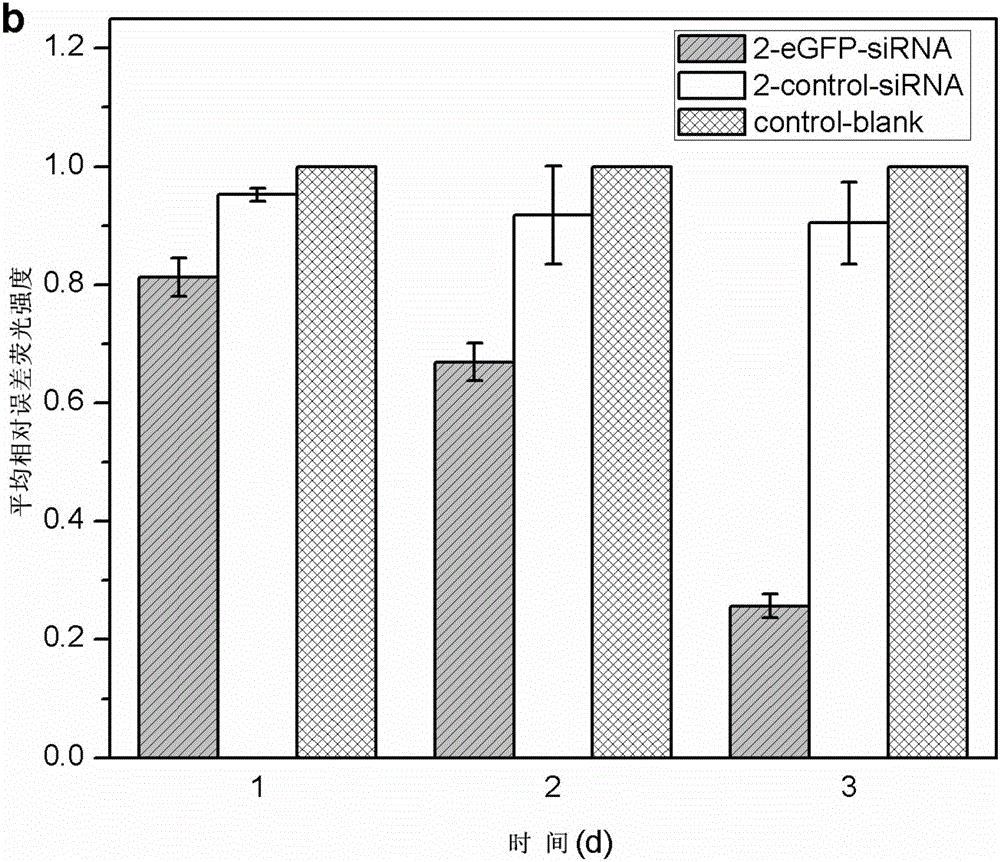
[0052] In a sterile workbench, prepare a 12 cm diameter petri dish with a built-in silicon wafer membrane of the same diameter (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A (1 mg / kg) of materials with cationic groups. ml chitosan, 0.1mol / L acetic acid, 0.2mol / L NaCl, pH=4) and liquid B (1mg / ml carboxymethyl chitosan, 0.15mol / L NaCl, pH=6) with anionic group material Take part B liquid and add small interfering nucleic acid drug (eGFP-siRNA (sense: 5'-GGCACAAGCUGGAGUACAAUU-3'; antisense: 5'-UUGUACUCCAGCUUGUGCCUU-3', 20ug / mL) and cell targeting factor (hyaluronic acid, molecular weight Less than 20,000 Daltons, 10ug / mL) and targeting peptide (1×10 -4 mg / ml) was formulated into liquid C, and the amino acid sequence of the targeted polypeptide was: valine-glycine-valine-alanine-proline-glycine; the above solutions were respectively filled into high-pressure instantaneous spraying equipment, first Spray liquid C into the petri dish with high pre...
Embodiment 2
[0054] In a sterile workbench, prepare a plate of polymer material (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A of materials with cationic groups (2 mg / ml chitosan, 0.1 mol / L acetic acid , 0.2mol / L NaCl, pH=4) and B solution (alginic acid of 2mg / ml, 60,000 to 80,000 molecular weight, 0.15mol / L NaCl, pH=6) with anionic group material is referred to as B solution; B solution Then add small interfering nucleic acid drug (same as Example 1, 20ug / mL) and cell targeting factor (hyaluronic acid, molecular weight is less than 20,000 Daltons, 10ug / mL), and then add targeting polypeptide (1 ×10 -4 mg / ml), the amino acid sequence of the targeted polypeptide is: isoleucine-lysine-valine-alanine-valine; first immerse the plate in solution A for 20 minutes, take it out into the cleaning solution, wash and dry; Then immerse the plate in solution B for 30 minutes, take it out, wash and dry it with cleaning solution, do this alternately 160 times, and finall...
Embodiment 3
[0056] In a sterile workbench, prepare a plate of polymer material (washed, sterilized and depyrogenated), and dry; respectively prepare solutions A of materials with cationic groups (2 mg / ml chitosan, 0.1 mol / L acetic acid , 0.2mol / L NaCl, pH=4) and B solution with anionic group material (2mg / ml hyaluronic acid, 0.15mol / L NaCl, pH=6) referred to as B solution; Nucleic acid cationic liposome (cationic lipid preparation method refers to "gene transfection mediated by anionic liposome-cationic liposome complex", Journal of Pharmaceutical Practice, 2011 (29): 4, small interfering nucleic acid is the same as Example 1 , small interfering nucleic acid cationic liposome addition amount is 0.5mg / ml, small nucleic acid drug load 21.7%) stir evenly and targeting polypeptide (1 * 10 -4 mg / ml), the amino acid sequence of the targeted polypeptide is: arginine-glycine-aspartic acid; first immerse the plate in solution A for 20 minutes, take it out into the cleaning solution, wash and dry; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com