Nitric oxide loaded cationic polymer, preparation method therefor and application of nitric oxide loaded cationic polymer
A cationic polymer, nitric oxide technology, applied in the field of biomedical engineering materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
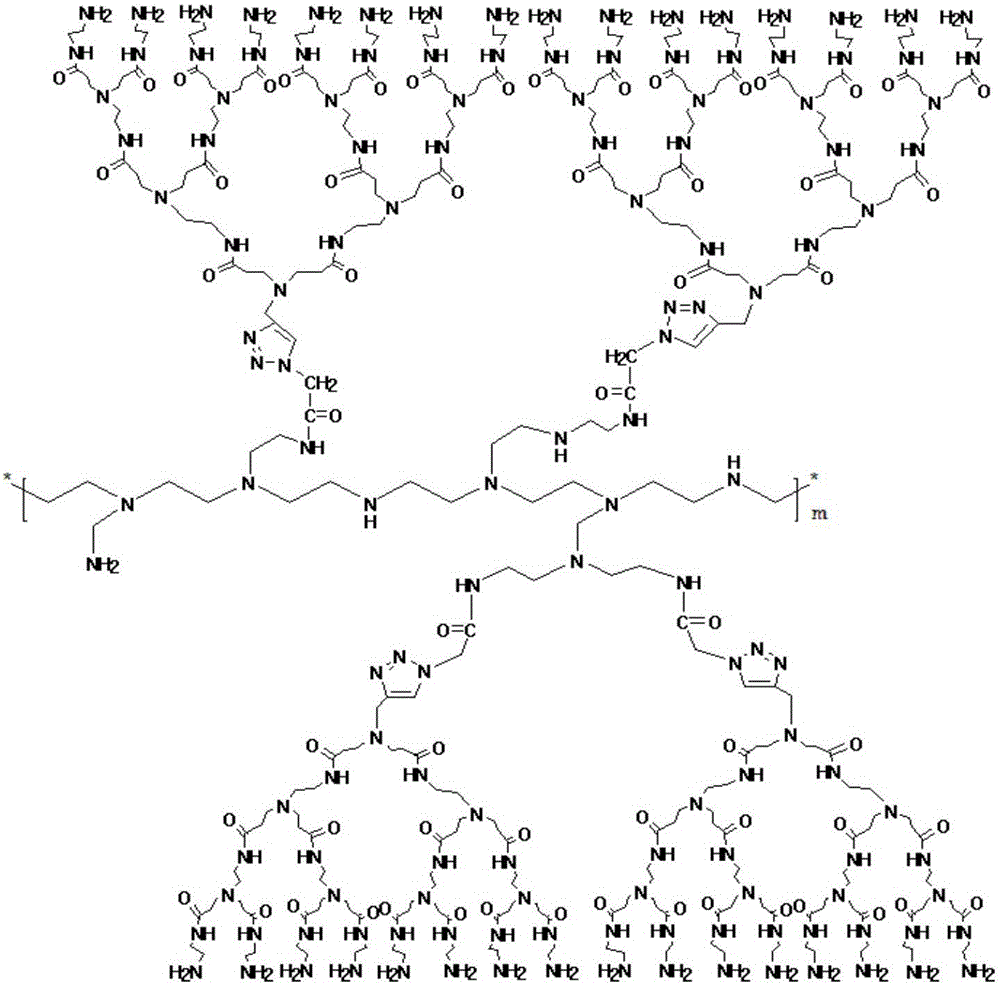
[0091] Embodiment 1: the synthesis of azide polyethyleneimine
[0092] Dissolve azidoacetic acid in pure water, add hyperbranched polyethyleneimine aqueous solution under ice bath, add N-hydroxysuccinimide (NHS) to stabilize it for 20 minutes, measure pH: 2, add 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) was reacted for 15 hours, dialyzed and freeze-dried to obtain azide-modified dendrimer polyethyleneimine (PEI-N 3 ).
[0093] The concentration of the polyethyleneimine aqueous solution is 20wt%; the weight average molecular weight of the PEI is 1800; the azidoacetic acid, polyethyleneimine, N-hydroxysuccinimide (NHS), 1-ethyl-( The molar ratio of 3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) is 10:1:1:1.
Embodiment 2
[0094] Embodiment 2: the synthesis of azide polyethyleneimine
[0095] Dissolve azidoacetic acid in pure water, add hyperbranched polyethyleneimine aqueous solution under ice bath, add N-hydroxysuccinimide (NHS) to stabilize for 30 minutes, measure pH: 3, add 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) was reacted for 18h, dialyzed and freeze-dried to obtain azide-modified dendritic polyethyleneimine (PEI-N 3 ).
[0096] The concentration of the polyethyleneimine aqueous solution is 30wt%; the weight average molecular weight of the PEI is 2000; the azidoacetic acid, polyethyleneimine, N-hydroxysuccinimide (NHS), 1-ethyl-( The molar ratio of 3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) is 11:1:1:1.
Embodiment 3
[0097] Embodiment 3: the synthesis of azide polyethyleneimine
[0098]Dissolve azidoacetic acid in pure water, add hyperbranched polyethyleneimine aqueous solution under ice-cooling, add N-hydroxysuccinimide (NHS) to stabilize for 40 minutes, measure pH: 5, add 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) was reacted for 20h, dialyzed and freeze-dried to obtain azide-modified dendrimer polyethyleneimine (PEI-N 3 )
[0099] The concentration of the polyethyleneimine aqueous solution is 50wt%; the weight average molecular weight of the PEI is 2500; the azidoacetic acid, polyethyleneimine, N-hydroxysuccinimide (NHS), 1-ethyl-( The molar ratio of 3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) is 12:1:1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com