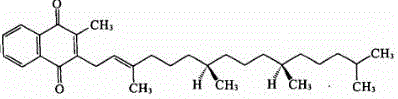
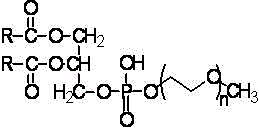
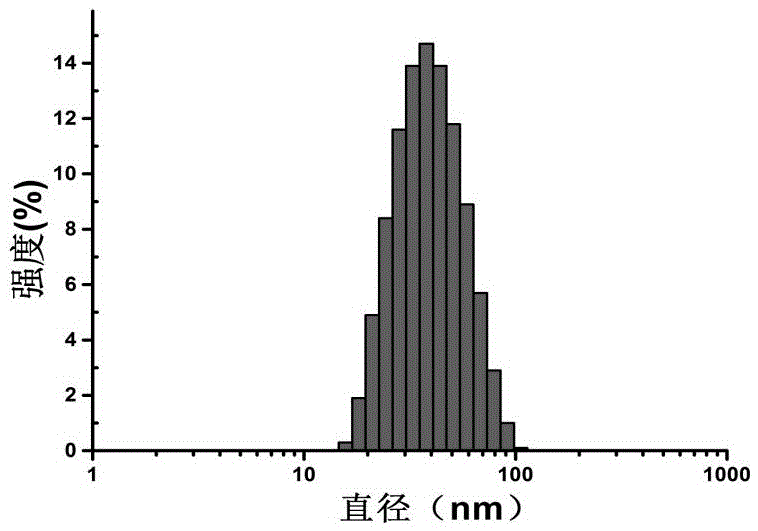
Vitamin K1 medicine with novel dosage form, and preparation method thereof
A vitamin and new dosage form technology, applied in the field of vitamin K1 micellar drug loading system and its preparation, can solve the problems of restricted application and high price, achieve high drug loading, less toxic and side effects, and avoid adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The invention provides a preparation method of vitamin K1-mPEG-PE, the steps are as follows:
[0018] (a). Preparation of mPEG-PE by chemical reaction: under the protection of nitrogen, add the tetrahydrofuran solution of diacylglycerol dropwise at 0°C to the tetrahydrofuran solution of diisopropylethylamine and phosphorus oxychloride, after the addition is complete Stirring was continued for 2 h. At 0° C., the solution of polyethylene glycol monomethyl ether and pyridine in tetrahydrofuran was continued to be added dropwise. After the addition was completed, the mixture was reacted at room temperature for 6 h. Filtrate, add water in an equimolar amount to phosphorus oxychloride to the filtrate, stir for 1-2 hours, evaporate the solvent to obtain a solid, and obtain mPEG-PE after precipitation and dialysis with diethyl ether.
[0019] (b). After mixing mPEG-PE and vitamin K1 in a certain ratio, add an appropriate amount of chloroform to dissolve, remove the chloroform b...
Embodiment 1
[0022] Example 1. Preparation of vitamin K1-mPEG-PE micelles by film hydration method
[0023] Precisely weigh 1g of vitamin K1 and 6g of mPEG-PE (prepared by the above chemical reaction) and dissolve them in 300mL of chloroform, and remove the chloroform by rotary evaporation to form a polymer drug mixed film, and dry it in vacuum overnight to remove the residual organic solvent . Add 100mL of water for injection, ultrasonicate in a water bath at 25°C for 40min, filter through a 0.22µm sterile microporous membrane, and freeze-dry to obtain the product.
Embodiment 2
[0024] Example 2. Preparation of vitamin K1-mPEG-PE micelles by film hydration method
[0025] Precisely weigh 1g of vitamin K1 and 6g of mPEG-PE (prepared by the above chemical reaction) and dissolve them in 300mL of chloroform, and remove the chloroform by rotary evaporation to form a polymer drug mixed film, and dry it in vacuum overnight to remove the residual organic solvent . Add 100mL of water for injection, ultrasonicate in a water bath at 37°C for 40min, filter through a 0.22µm sterile microporous membrane, and freeze-dry to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com