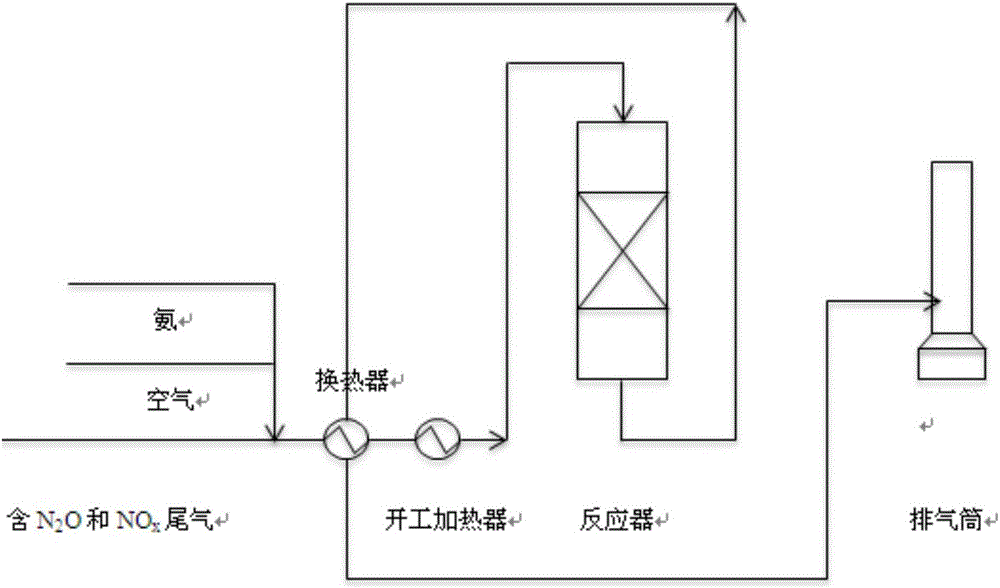
Method for simultaneously removing N2O and NO
A removal and integration technology, applied in the field of removal of N2O and NOx, can solve the problems of complex process, large investment in equipment and materials, and achieve the effect of simple process, saving equipment investment and simplified operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
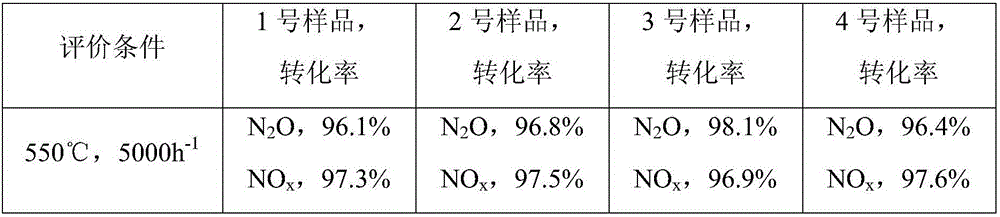
Image
Examples
Embodiment 1
[0042] The catalyst is produced according to the following preparation process
[0043] (1) Impregnation: 10 grams of ammonium metavanadate and 5 grams of cobalt oxalate were added to 60 milliliters of ammonia solution in a volume ratio of 1:10, dissolved completely, and the solution was impregnated on 100 grams of alumina carrier in equal amounts.
[0044] (2) Drying: the impregnated alumina carrier is dried in the shade and then dried by hot air at 120°C.
[0045] (3) Roasting: The semi-finished product after drying is roasted at 550°C to fully decompose the salt.
[0046] (4) secondary dipping: after the semi-finished product after roasting is naturally cooled, 1.6 grams of palladium chloride is dissolved in 60 milliliters of 1:10 hydrochloric acid solution in a volume ratio, and the dissolution is complete, and the solution is immersed in the carrier of step (3) in equal amounts superior.
[0047] (5) Reduction of precious metals: The carrier impregnated with precious me...
Embodiment 2
[0053] The catalyst is produced according to the following preparation process
[0054] (1) Impregnation: 14 grams of copper acetate monohydrate and 8 grams of zinc acetate dihydrate were added to 60 ml of acetic acid solution in a volume ratio of 1:10, and the solution was completely dissolved, and the solution was impregnated on 100 grams of alumina carrier in equal amounts.
[0055] (2) Drying: the impregnated alumina carrier is dried in the shade and then dried by hot air at 120°C.
[0056] (3) Roasting: The semi-finished product after drying is roasted at 450°C to fully decompose the salt.
[0057] (4) Secondary immersion: after the semi-finished product after roasting is naturally cooled, 5.2 grams of chloroplatinic acid is dissolved in 60 ml of a hydrochloric acid solution with a volume ratio of 1:10, the dissolution is complete, and the solution is immersed in the carrier of step (3) in equal amounts. superior.
[0058] (5) Reduction of precious metals: The carrier i...
Embodiment 3
[0064] The catalyst is produced according to the following preparation process
[0065] (1) Impregnation: 6 grams of ammonium metavanadate and 3.5 grams of copper acetate were added to 60 milliliters of ammonia solution with a volume ratio of 1:10, and the solution was completely dissolved, and the solution was impregnated on 100 grams of alumina carrier in equal amounts.
[0066] (2) Drying: the impregnated alumina carrier is dried in the shade and then dried by hot air at 120°C.
[0067] (3) Roasting: The semi-finished product after drying is roasted at 550°C to fully decompose the salt.
[0068] (4) Secondary impregnation: after the semi-finished product after roasting is naturally cooled, 13.7 grams of rhodium nitrate is dissolved in 60 milliliters of water, and the dissolution is complete, and the solution is impregnated on the carrier of step (3).
[0069] (5) Reduction of precious metal: The carrier impregnated with precious metal uses ascorbic acid as a reducing agent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com