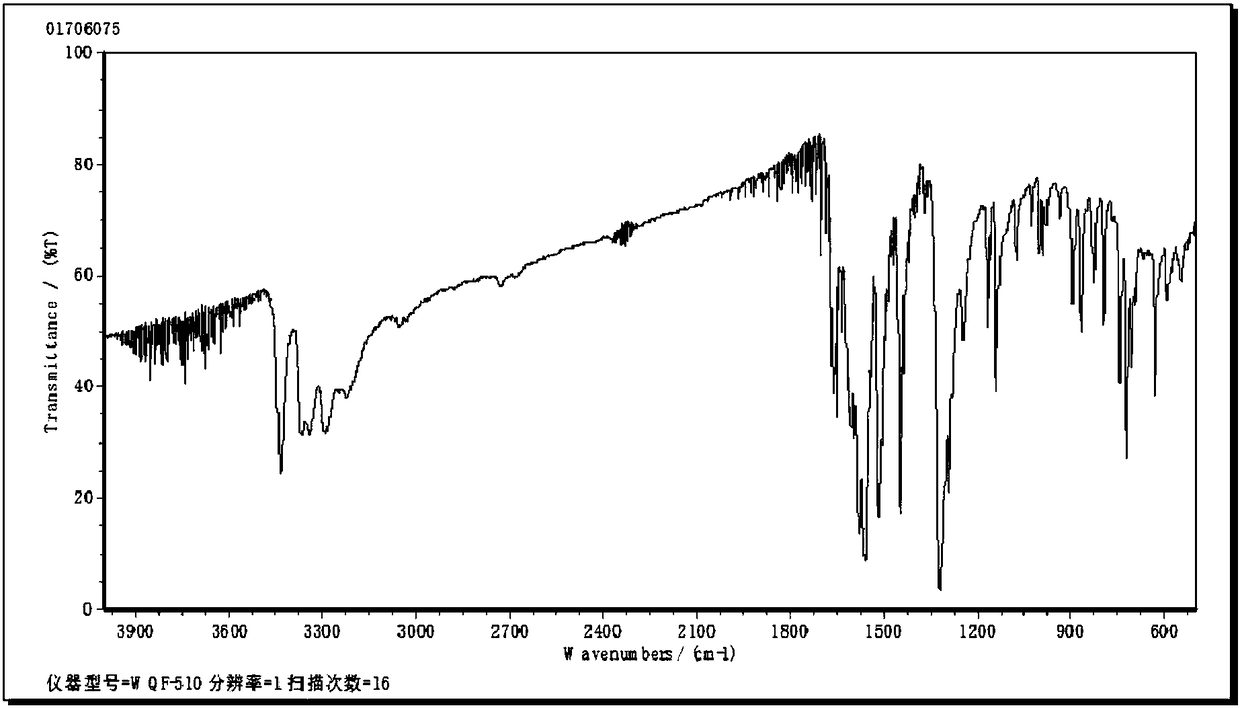
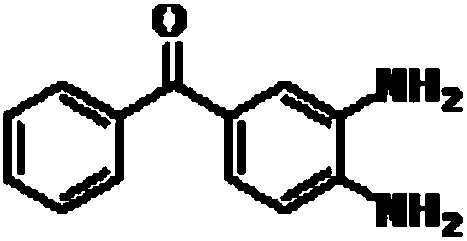
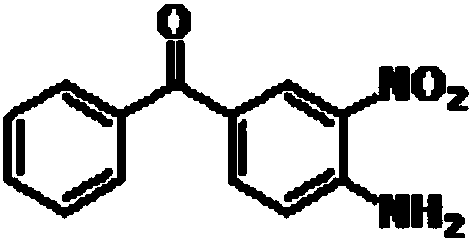
A kind of preparation method of mebendazole intermediate 3,4-diaminobenzophenone
A technology of diaminobenzophenone and nitrobenzophenone, which is applied in the field of preparation of mebendazole intermediate 3,4-diaminobenzophenone, can solve the problem of high operating pressure and side effects of catalytic hydrogenation Problems such as large amount of product and decrease in product purity are achieved to overcome relatively high requirements for reaction control, short reaction time, and less solvent usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 5
[0035] Embodiment 1-embodiment 5 relates to the preparation of Pd-X / C catalyst
Embodiment 1
[0037] Preparation of Pd-Ru / C catalyst: 0.135g H 2 PdCl 4 and 0.03g RuCl 3 Dissolve in 30mL of water to form a solution. Immerse 1g of activated carbon in the solution for 10 hours, then heat the solution to 95°C, add 2.0g of phosphorous acid, heat and reduce for 1 hour, cool after the reduction, filter and wash with water To neutrality, the Pd-Ru / C catalyst is prepared.
[0038] After testing, the obtained Pd-Ru / C catalyst has a specific surface area of 1120m 2 / g, water content 40%, strength 95%, impurity 0.2%.
Embodiment 2
[0040] Preparation of Pd-Ti / C catalyst: 0.135g H 2 PdCl 4 and 0.4g TiCl 4 Dissolve in 30mL of water to form an aqueous solution; soak 1g of activated carbon in the aqueous solution for 12 hours, heat to 90°C, add 1.0g of CH 3 COONa, heated and reduced for 1.5 hours, cooled after the reduction was completed, filtered, and washed with water until neutral to obtain a Pd-Ti / C catalyst.
[0041] After testing, the obtained Pd-Ru / C catalyst has a specific surface area of 1210m 2 / g, 50% water content, 92% strength, 0.2% impurity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com