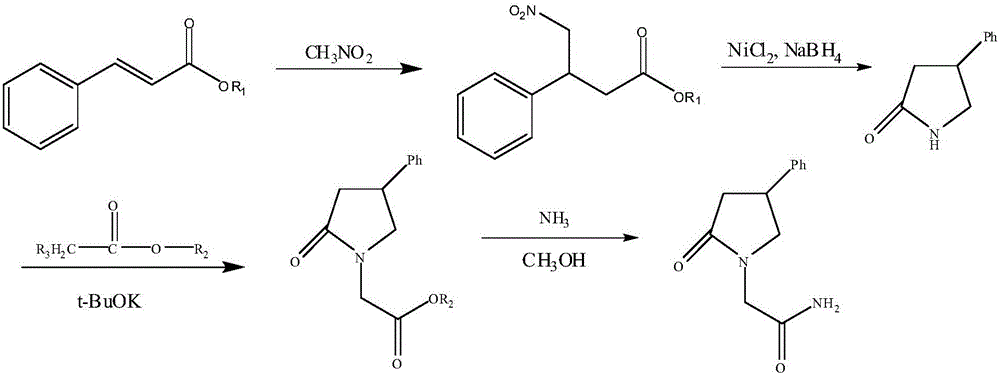
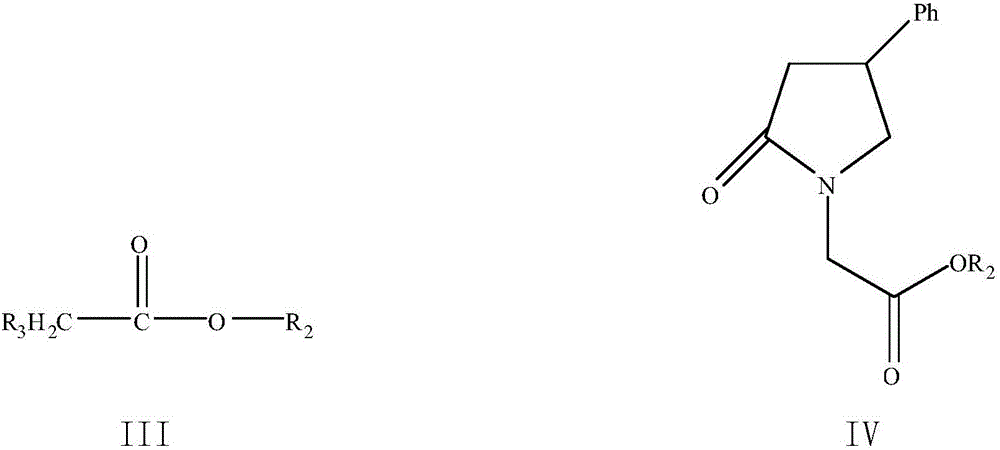Method for preparing phenyl piracetam
A technology of phenylpiracan and benzene, which is used in the field of compound preparation, can solve problems such as low molecular utilization, complex operation, and long response steps to achieve high reaction income and purity, high atomic utilization, response, response, response, response, response, The effect of short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
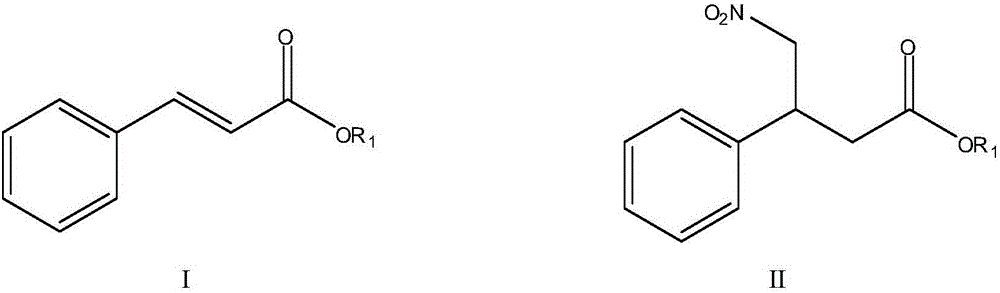
[0023] Preparation of ethyl 4-nitro-3-phenylbutyrate
[0024] (1) The influence of different molar ratios of raw materials on the reaction yield:
[0025] Add 200mL of anhydrous methanol, ethyl cinnamate, and nitromethane into the three-necked flask, and slowly add cold 30% NaOH aqueous solution dropwise below 0°C. The selection of different molar ratios of the raw materials is shown in Table 1, 20-30°C React for 24 hours to obtain a reaction solution; concentrate the reaction solution at 40-50°C under reduced pressure to evaporate the organic solvent, add 100ml of water, extract the water phase twice with 80ml of dichloromethane, combine the dichloromethane layers, and wash with 40ml of 1N hydrochloric acid Wash once, and then wash once with 40ml of water to obtain an organic phase extract; add 8g of anhydrous magnesium sulfate to the organic phase extract and dry for 1-2h, filter, and distill off the solvent under reduced pressure to obtain a light yellow oil, namely 4-Nitra...
Embodiment 2
[0037] Preparation of 4-phenyl-2-pyrrolidone
[0038] (1) Adopting the impact of different reduction methods on the reaction yield:
[0039] Dissolve 16.6g (0.07mol) of ethyl 4-nitro-3-phenylbutyrate in 250mL of dichloromethane, stir and cool down to -10°C, slowly add 0.35mol of reducing agent, after the addition is complete, rise to 15-30°C , timed and stirred for 5 hours to obtain the reaction solution. The selection of the reducing agent is shown in Table 1; the reaction solution was cooled to 0°C, 550mL of 10% dilute hydrochloric acid and 550mL of dichloromethane were added, quenched by stirring at 20-30°C, and clarified Extract the aqueous layer with 90mL×2 dichloromethane, combine the dichloromethane layers, and wash three times with 220mL×3 20% aqueous sodium chloride solution; add 8g of anhydrous magnesium sulfate to the organic phase extract and dry for 1-2h , filtered, and dichloromethane was evaporated under reduced pressure to obtain beige solid 4-phenyl-2-pyrroli...
Embodiment 3
[0052] Preparation of 4-phenyl-2-pyrrolidone-1-acetic acid methyl ester
[0053] (1) Adopting the impact of different organic solvents on the reaction yield:
[0054] Dissolve 9.6 g (0.06 mol) of 4-phenyl-2-pyrrolidone in 100 mL of an organic solvent. The selection of the organic solvent is shown in Table 1. Cool down to an internal temperature of -10° C., stir, and add 13.5 g (0.12 mol) Potassium tert-butoxide, after the addition, continue to stir at -10-0°C for 2h, cool down to the internal temperature -10°C, add 13.0g (0.12mol) methyl chloroacetate dropwise, and continue to react at -10-0°C for 2h, After the reaction is complete, add 160mL ethyl acetate, 65mL saturated ammonium chloride / 65mL water, stir and let stand to separate layers, extract the water layer twice with 80mL×2 ethyl acetate, combine the organic layers, wash 2 times with 40mL×2 saturated ammonium chloride Once, washed with 40mL of water once, dried with 8g of anhydrous magnesium sulfate for 30min, filtered...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com