Synthesis method of 2-(1-hydroxy-4-keto-2,5-cyclohexadiene)-pyran-4-ketone
A technology of cyclohexadiene and a synthesis method, which is applied in the field of pharmaceutical synthesis, can solve the problems of high price of 4-ethynyl anisole, difficult operation and danger, many side reactions of cyclization, etc., and achieves cheap raw materials, equipment and reaction. Low-condition, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
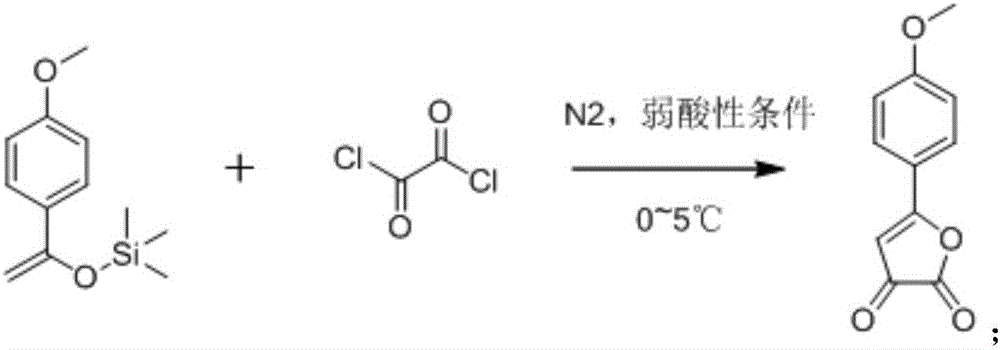
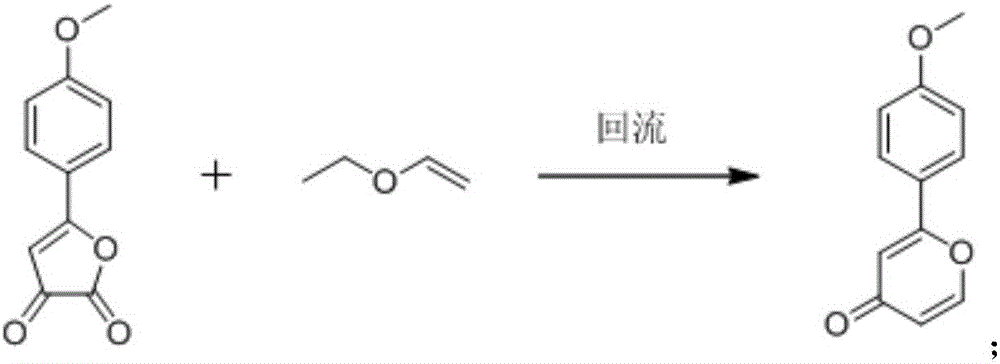
[0028] A kind of synthetic method of 2-(1-hydroxyl-4-ketone-2,5-cyclohexadiene)-pyran-4-one, its step is:
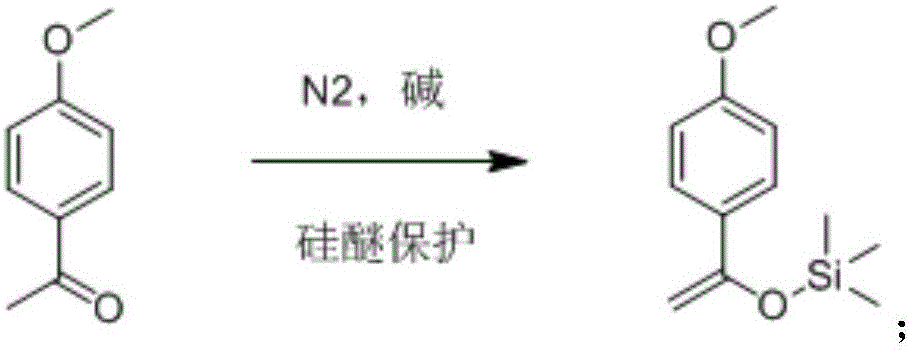
[0029] 1) Synthesis of 1-(4-methoxyphenyl)-1-trimethylsiloxyethylene:
[0030] in N 2 Under protection, 10g (69.59mmol) of p-methoxyacetophenone and 16.85g (146.5mmol) of triethylamine were dissolved in 200ml of acetonitrile, and slowly added dropwise to the above mixed solution (dropping was completed within 15min) 15.92g ( 146.5mmol) trimethylchlorosilane, stirring and reacting at room temperature for 3h, after the completion of the reaction, stop N 2 protection, slowly add 150mL saturated NH 4 The Cl solution was extracted three times with 50 ml of n-hexane. After the extraction was completed, the obtained organic phase was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated and dried to obtain 12.7 g of a brown oily liquid with a yield of 86.0%. TLC: Rf=0.6 (petroleum ether / ethyl acetate=10 / 1). The crude product obtained by this treatm...
Embodiment 2
[0049] A kind of synthetic method of 2-(1-hydroxyl-4-ketone-2,5-cyclohexadiene)-pyran-4-one, its step is:
[0050] 1) Synthesis of 1-(4-methoxyphenyl)-1-trimethylsiloxyethylene:
[0051] in N 2 Under protection, 17.7g (175mmol) of diisopropylamine was dissolved in anhydrous tetrahydrofuran, and the temperature was lowered to -45~-40°C, and then 76ml of 2.2M n-butyllithium was slowly added dropwise, and the reaction was carried out at this temperature for 20~30min. Then naturally raise the temperature to room temperature and react for 1.5~2h. After the reaction is completed, cool down to -45~-40°C, slowly add 10g (66.59mmol) p-methoxyacetophenone, stir for 10min, then slowly add 14.5g (133.2 mmol) trimethylchlorosilane, stirring reaction at room temperature for 3h, after the completion of the reaction, stop N 2 protection, slowly add 220mL saturated NH 4 The Cl solution was extracted three times with 60 ml of n-hexane. After the extraction was completed, the obtained organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com