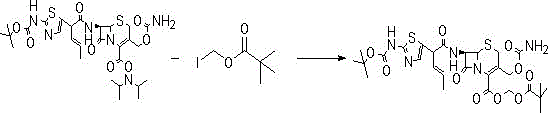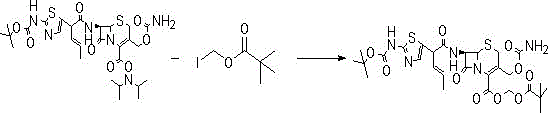Method for preparing cefcapene pivoxil hydrochloride
A technology of cefcapene hydrochloride and iodomethyl pivalate, which is applied in the field of medicine and chemical industry, can solve the problems of low purity and low yield of cefcapene hydrochloride, and achieve the advantages of purity, short route and simple synthesis operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
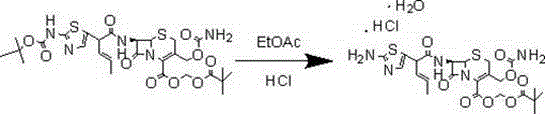
[0023] Embodiment 1: the synthesis of cefcapene pivoxil hydrochloride
[0024] Add 200g of dichloroethane into the three-necked flask, add 20g of BCN under stirring, stir at room temperature 25°C, then start to cool down to -25°C~-20°C, start to add 25.7g of iodine ester dropwise at T=-20°C, keep at -25 ℃~-20℃ and stirred for 3h.
[0025] Post-treatment: Add 200g EA and 300g water to the system, stir and extract at room temperature 25°C; separate layers, and extract the water layer with 100EA again. Combine the EA layers, wash the EA layer once with 300g of saturated sodium bicarbonate, wash the EA layer once with 350g of saturated saline, dry and decolorize the EA layer, concentrate to a solid, add 2 times of EA after weighing, dissolve it, and drop it into 400g of petroleum ether Stir and precipitate, and filter to obtain light yellow solid powder.
[0026] Obtain (Ⅱ) compound, yield: 85%~90%, LC: 99%;
[0027] Under the protection of nitrogen, add 200ml of hydrogen chlor...
Embodiment 2
[0029] Embodiment 2 The identification of product in the embodiment of the present invention 1
[0030] Identification method: Bruker Avance III 400MHz superconducting NMR spectrometer
[0031] Analysis method: LC (liquid chromatography purity), Shimadzu LC-10AT VP, Shimadzu C-18 chromatographic column,
[0032] Mobile phase: acetonitrile: water = 65:35, flow rate 2ml / min.
[0033] 1H NMR ( 500 MHz, ( CD3 ) 2 SO) δ : 0. 97 ~ 1. 01 ( m, 3H), 1. 15 ( s, 9H), 2. 12 ~ 2. 21 ( m, 2H), 3 .50( d, J = 18. 5 Hz, 1H), 3. 60 ( d, J = 18. 5Hz, 1H), 4. 55 ( d, J = 13. 0Hz, 1H), 4. 79 ( d , J = 13. 0 Hz, 1H ), 17( d, J = 5.0 Hz, 1H), 5. 79 ~ 5. 82( m, 2H), 5. 87( d, J = 5. 0 Hz, 1H ), 6. 23( t, J = 7. 5Hz, 1H), 6. 51 ~ 73( m, 2H), 7. 14( s, 1H), 9. 27( d, J = 8. 0Hz, 1H ).
[0034] 7-[2-(2-amino-1,3-thiazol-4-yl)pent-3-enamido]-3-(carbamoyloxymethyl)-8-oxo-5 provided by the present invention -Compared with other synthetic methods, the synthetic method of -thio-1-azabicyclo[4.2.0]oct-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com