An efficient near infrared fluorescent material and biological applications thereof
A near-infrared and fluorescent dye technology, applied in the application fields of high-efficiency near-infrared fluorescent dyes, bioimaging and fluorescent labeling, can solve the problems of fluorescence analysis technology measurement error, decrease in fluorescence intensity, dye self-quenching, etc., and achieve high solid state Effects of fluorescence quantum efficiency and resistance to photobleaching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of precursors
[0022] Compound 1:
[0023] p-Bromophenylacetonitrile (5g, 25.505mmol) and iodine (6.5734g, 25.505mmol) were placed in a 250mL reaction bottle, replaced by argon three times until there was no oxygen and water in the bottle, and 100mL of dry ether was added and stirred to dissolve. Dissolve sodium methoxide (2.8933g, 5.356mmol) in anhydrous methanol (8.680g, 14.613ml), deoxygenate and water, slowly drop into the above solution at -78 degrees Celsius, and keep Argon atmosphere. After the dropwise addition, the temperature was slowly raised to 0°C, and the stirring reaction was continued for 3 hours. After the reaction was completed, the reaction was quenched with 5% dilute hydrochloric acid solution, and then stirred for 12 hours. After the reaction was complete, the solution was filtered with suction, and the filter cake was washed twice with methanol and water. The resulting solid was dried in a vacuum oven. The final product was compou...
Embodiment 1
[0027] Take compound 2 (159.6mg, 0.52mM), p-dianilinostyrene (140mg, 0.52mM), Pd(OAc) respectively 2 (6mg, 0.026mM), Ag 2 CO 3 (85 mg, 0.31 mM) was added to a 50 mL Schlenk tube, about 10 mL of toluene was added, and the reaction was heated at 100 degrees Celsius for 18 hours. After the reaction, the reaction solution was filtered, the filtrate was spin-dried to dry the solvent, and purified by a silica gel column to obtain a deep red powder product compound 3 with a yield of 53.4%. 1HNMR (500MHz, DMSO) δ7.82 (ddd, J = 13.8, 8.6, 6.0Hz, 9H), 7.55 (d, J = 8.7Hz, 2H), 7.43 (s, 1H), 7.39 (s, 1H), 7.33(dd, J=8.3, 7.5Hz, 4H), 7.22(s, 1H), 7.19(s, 1H), 7.11–7.03(m, 6H), 6.96(d, J=8.6Hz, 2H).Ms (m / e): C 36 h 25 N 3 , calcd499.6; found499.6. Elemental analysis experimental value (calculated value): C: 86.6% (86.55%); H: 5.0% (5.04%); N: 8.4% (8.41%).
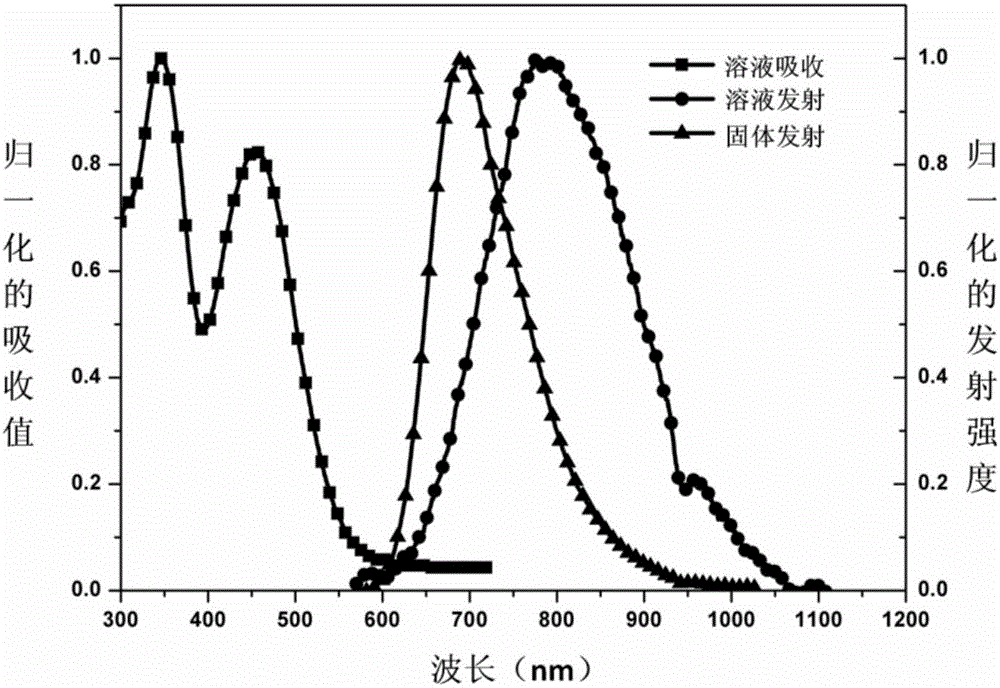
[0028] Depend on figure 1 It can be seen that the maximum absorption peak of the tetrahydrofuran solution of compound 3 is 45...
Embodiment 2
[0032] Take compound 1 (200mg, 0.52mM), p-dianilinostyrene (280mg, 1.04mM), Pd(OAc) respectively 2 (12mg, 0.052mM), Ag 2 CO 3 (170 mg, 0.62 mM) was added to a 50 mL Schlenk tube, about 10 mL of toluene was added, and the reaction was heated at 100 degrees Celsius for 18 hours. After the reaction was completed, the reaction solution was filtered, the filtrate was spin-dried to dry the solvent, and purified by a silica gel column to obtain compound 4 as a dark red powder with a yield of 62.8%. 1 HNMR (500MHz, DMSO) δ7.87(d, J=8.6Hz, 4H), 7.82(d, J=8.6Hz, 4H), 7.58(d, J=8.8Hz, 4H), 7.43(d, J=8.6Hz, 4H) 16.3Hz, 4H), 7.38–7.33(m, 8H), 7.23(d, J=16.5Hz, 4H), 7.13–7.06(m, 11H), 6.98(d, J=8.7Hz, 4H).Ms( m / e): C 56 h 40 N 4 , calcd768.33; found767.136. Elemental analysis experimental value (calculated value): C: 87.5% (87.47%); H: 5.2% (5.24%); N: 7.3% (7.29%).
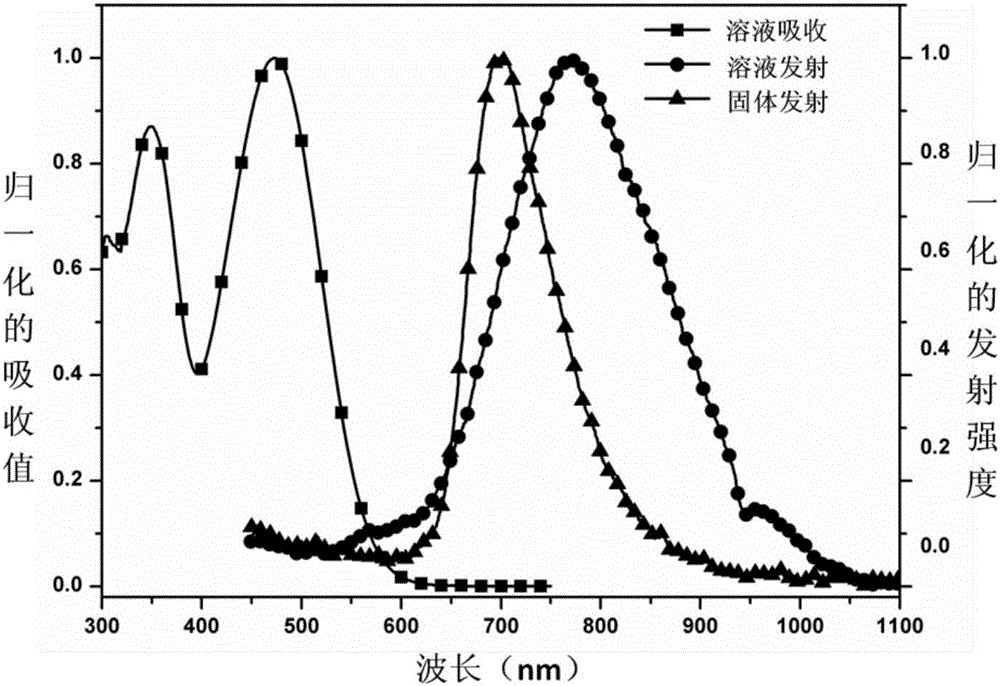
[0033] Depend on figure 2 It can be seen that the maximum absorption peak of compound 4 is 472nm, and the maximum e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com