Application of compound pseudoephedrine hydrochloride tablet to preparation of medicine for treating asthma
A technology of compound prescription and Fen tablets, applied in the field of medicine, can solve problems such as hypokalemia, cardiac rhythm disorder, single use, and excessive application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 compound pseudomamefen tablets
[0018] (1) Pseudoephedrine hydrochloride, dextromethorphan hydrobromide, diphenhydramine hydrochloride, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone, magnesium stearate and dextrin were pulverized respectively, and passed through a 40-mesh sieve;
[0019] (2) Mix 3 g of pseudoephedrine hydrochloride, 1.5 g of dextromethorphan hydrobromide, 2.5 g of diphenhydramine hydrochloride, 0.75 g of low-substituted hydroxypropyl cellulose, 0.45 g of polyvinylpyrrolidone, and 6.5 g of dextrin;
[0020] (3) 3g magnesium stearate is added in the mixture of step (2), mix homogeneously;
[0021] (4) the mixture that step (3) obtains with 10kg / mm 2 Compression tableting, prepare 100 compound pseudo-mamefene tablets.
Embodiment 2
[0022] The preparation of embodiment 2 compound pseudomamefen tablets
[0023] (1) Pseudoephedrine hydrochloride, dextromethorphan hydrobromide, diphenhydramine hydrochloride, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone, magnesium stearate and dextrin were pulverized respectively, and passed through a 40-mesh sieve;
[0024] (2) Mix 3 g of pseudoephedrine hydrochloride, 1.5 g of dextromethorphan hydrobromide, 2.5 g of diphenhydramine hydrochloride, 0.75 g of low-substituted hydroxypropyl cellulose, 0.45 g of polyvinylpyrrolidone, and 6.5 g of dextrin;
[0025] (3) 3g magnesium stearate is added in the mixture of step (2), mix homogeneously;
[0026] (4) the mixture that step (3) obtains with 8kg / mm 2 Compression tableting, prepare 100 compound pseudo-mamefene tablets.
Embodiment 3
[0027] Non-clinical pharmacodynamics study of embodiment 3 compound pseudomamefen tablets
[0028] 1. Experimental materials
[0029] 1.1 Experimental animals
[0030] Sexually mature and healthy male Kunming mice, with an average age of 8 weeks, were purchased from Hubei Provincial Center for Disease Control and Prevention. The mice were kept at room temperature of 20-25° C. and humidity of 50-60%, and the light-dark cycle interval was 12 hours.
[0031] 1.2 Experimental instruments and reagents
[0032] Compound Pseudomamefen Tablets (self-made); Absolute Ethanol (Sinopharm Chemical Reagent Co., Ltd.);
[0033] Nifedipine (U.S. Sigma Company); Pyrazole 3 (U.S. Sigma Company);
[0034] Acetylcholine (Sigma, USA);
[0035] Stereo microscope (Wuhan Aispei Scientific Instrument Co., Ltd.);
[0036] Ice machine (Chongzhou Shubo Chemical Co., Ltd.);
[0037] Erlenmeyer flask (Chongzhou Shubo Chemical Co., Ltd.);
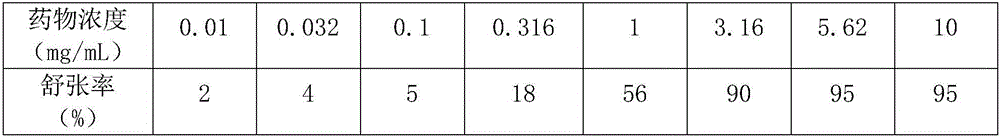
[0038] HV-4 type constant temperature perfusion system for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com